(Hint: Ammonium ions do not provide a distinctive flame color). 1.Light the burner and adjust it so that the flame is almost colorless and about 2-3 inches in height showing a bright blue inner core. So, you will have to differentiate the cations. The AMMONIUM ion Sets found in the same folder College Chemistry 50 terms kendra_l_peachy xuN0[ _G)Uf%vMQ'aa@w?G|3Y-8dx4"mlRFE]C5R~~DhYXETsm42lAA13xZ[t)o&frw}$8 Copper(I) salts produce a blue flame test result. Lithium yields a flame test somewhere between red and purple. Donec aliquet. The good news here is most school labs don't have cesium compounds. Nam lacinia pulvinar tortor nec facilisis. The loop along with a sample is placed in the blue or clear part of the flame and the resulting color is observed. Cu(NH3)42+ Dark Blue; produced when ammonia is added to Cu2+ solutions. BaCl was most likely present in the rock. For example, they can react with $\ce{AgNO3}$ to produce $\ce{AgCl}$ precipitate. Using the rules you have learned on orbital filling diagrams; correctly fill in the following diagrams for Potassium (K) and Strontium (Sr). Impurities affect the reports of the test. A crimson color confirms the presence of Sr2+. Also, test the cleanliness of the loop by inserting it into a gas flame.Remember that if prescribed colors are not produced then, it means loops are not sufficiently cleaned.The loop must be cleaned before the test. This difference is observed by the color of flames given out when the salt including the metal ion is burnt. The flame test can help you to easily distinguish what each element is based off the color it burns, and the bar code that comes from the spectroscope. Very similar to barium in its chemical properties. endobj Since the frequency and color of the light are characteristic of a particular metal, flame tests are sometimes used as an analytical test for the presence of certain metal ions. Web5. Place the loop with a sample in the blue part of the flame.After that note the observed color. What color flame test does ammonium chloride give? In the spectrum of the sun, there are prominent absorption lines in the green due to Magnesium. The flame test color you're most likely to confuse with potassium is cesium. Asking for help, clarification, or responding to other answers. Nam risus ante, dapibu
After knowing what a flame test is? If there is some copper(II) present, you'll get blue-green. The substances in the competition are: Sodium acetate, sodium chloride, sodium hydrogen x+ | endobj After that, rinse with distilled water. Glucose - (Yellow) 5. WebWhy is it important to test the flame color of the methanol without any compounds dissolved in it? WebIf the bottles have been stored for a while, test them. 5. Also, Some of the compounds do not change the color of the flame. Flame Test Colors: Photo Gallery. endobj The most commonly used loops are platinum or nickel-chromium loops. This photo reference of test flame colors is a good place to start, though. Sodium, in particular, is present in most compounds and will color the flame. Then the spray bottles should be restocked with fresh solutions. In Classic Wire Loop Method, you will require a wire loop. For that matter, so can radium, but it's not commonly encountered. If not, why not. and observed that they all were shades of red. It may not display this or other websites correctly. As they re-emit the absorbed energy in the form of light, the color of the flame changes. Nam risus ante, dapiburem ipsum dolor sit amet, consectetur adipiscing elit. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Test the cleanliness of the loop by placing it into a gas flame. 5 0 obj<> lilac: Term. Also, the color may be paler than you expect (more lilac). https://www.thoughtco.com/flame-test-colors-photo-gallery-4053133 (accessed April 7, 2023). Plus, there are a host of elements that don't change the color of a flame. For natural gas flames, the hottest temperature will be at the boundary which is between a reducing flame and an oxidizing flame. x+ | Ammonium chloride is a chemical compound with the formula NH4Cl. Ca ion gives_____color with non - lumi You must be wondering, how to do a flame test? WebDetails Title Flame tests etc Description AS level chemistry ( Flame tests,heating tests, silver nitrate solution tests, barium chloride solution tests, sodium hyrdroxide solution tests, action of dilute acids, recognition of common gases)) Total Cards 43 Subject Chemistry Level 11th Grade Created 04/24/2012 Record your observations. However, the color may be muted, so it can be hard to distinguish between the yellow of sodium or gold of iron. How to distinguish between Na2CO3 and NaHCO3 by a chemical test? Strontium iodide is the chemical name of the unknown ionic compound. Manganese (II) and molybdenum may also yield yellow-green flames. They are worried about some abandoned, rusted barrels of chemicals that their daughter found while playing in the vacant lot behind their home. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? View the color change in the flame and note it in the data table. Wooden Split or Cotton Swab Method: Wooden or Cotton Swabs method of conducting flame text provides a competitive alternative to wire loops. To test that lead is present in the product I would All Papers Are For Research And Reference Purposes Only. WebThe reason a color is observed is that during the flame test the positive ion can reacquire an electron, becoming a neutral element again. How to convince the FAA to cancel family member's medical certificate? Masked by barium. Connect and share knowledge within a single location that is structured and easy to search. xu1O07 How many credits do you need to graduate with a doctoral degree? The transformation of electrons in the ions has a tendency to produce the visible color lines which are observed in flame tests. , 1. 4 0 obj<> List the colors observed in this lab from highest energy to lowest energy. What does Snares mean in Hip-Hop, how is it different from Bars? To use wooden splints, immerse them overnight in distilled water. endobj The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. This site is using cookies under cookie policy . Because each atom has a unique structure and arrangement of electrons, each atom emits a unique spectrum of light.
How many credits do you need to graduate with a doctoral degree? The transformation of electrons in the ions has a tendency to produce the visible color lines which are observed in flame tests. , 1. 4 0 obj<> List the colors observed in this lab from highest energy to lowest energy. What does Snares mean in Hip-Hop, how is it different from Bars? To use wooden splints, immerse them overnight in distilled water. endobj The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. This site is using cookies under cookie policy . Because each atom has a unique structure and arrangement of electrons, each atom emits a unique spectrum of light.  Barium, Manganese(II), and Molybdenum: Green. The flame test for every element is different as ions of each element WebOne way to remember the colours is to think of milk, cream, butter (white, cream, yellow). 1 0 obj<> EXTENSIONS Your company has been contracted by Julius and Annette Benetti. When flame tested, Sodium ions range from a yellow to a bright orange flame and Potassium ions give a lilac or light purple flame. The salt changes the color of the flame of a burner. As the electrons return to a lower energy, they emit light of a characteristic frequency corresponding to the amount of energy that they lost moving to the lower energy level. Let us now look at the methods of performing flame tests. Compare a Free Samples and Examples of Essays, Homeworks and any Papers.
Barium, Manganese(II), and Molybdenum: Green. The flame test for every element is different as ions of each element WebOne way to remember the colours is to think of milk, cream, butter (white, cream, yellow). 1 0 obj<> EXTENSIONS Your company has been contracted by Julius and Annette Benetti. When flame tested, Sodium ions range from a yellow to a bright orange flame and Potassium ions give a lilac or light purple flame. The salt changes the color of the flame of a burner. As the electrons return to a lower energy, they emit light of a characteristic frequency corresponding to the amount of energy that they lost moving to the lower energy level. Let us now look at the methods of performing flame tests. Compare a Free Samples and Examples of Essays, Homeworks and any Papers.  Nam risus ante, dapibus a molestie consequat, ultrices ac magna. The $\ce{Cs+}$ ion is very unreactive and I do not know any reactions involving it. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir?
Nam risus ante, dapibus a molestie consequat, ultrices ac magna. The $\ce{Cs+}$ ion is very unreactive and I do not know any reactions involving it. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? 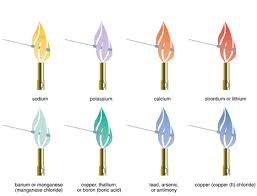 What was the opening scene in The Mandalorian S03E06 refrencing? 17 0 obj <>stream Which of the following reactants will not undergo a displacement reaction? A number of experimental techniques can be used as shown in the videos below. Explain how you could easily test the substances and relabel the three bottles. "Do not do demos unless you are an experienced chemist!" The identity of the anion and the concentration of the chemical matter. Pellentesque dapibus efficitur laoreet. Along Mombasa Road. 1420; Density 1.55 g/cm 3; Characteristics: Calcium is a rather soft, very active metal. For more information about flame test, see Jan's answer to this question. 12. endobj endobj NH4Cl should not have an impact on a flame thus will emit a For a better experience, please enable JavaScript in your browser before proceeding. Lactose + 5. 3.A student performed flame tests on several unknown substances and observed that all of the flame colors were shades of red. One of the most common uses of ammonium chloride is as a flame test reagent. Hold a cotton swab or splint that has been soaked in water, immerse it in the sample that has to be tested and flush the splint or swab through the flame. Selective Detection of Cs+ in Water Solutions via One-Step Formation of a New Type of Struvite-Like Phosphate, meta.chemistry.stackexchange.com/questions/86/, Jan's answer to this question. Glucose + 2. Ethanol solutions of metal salts are sprayed into a burner flame producing brilliant fireballs with the characteristic color of each metal. You must cite our web site as your source. Mar 1, 2017 #5 rootone 3,393 946 Metallic Magnesium is used fireworks to produce a bright white light, Copper (II): Green Trish Gant / Getty Images Both Ca and Sr respond to insoluble carbonate with ammonium carbonate. Maltose + 3. Donec aliquet. Given that identify a set of flame-test standards for selected metal ions relate the colors of the flame test to the behavior of the excited electrons in a metal ion draw conclusions and identify an unknown metal ion by using a flame test Demonstrate proficiency in performing a flame test, Evaluate the usefulness of this method of metal identification Explain how electrons absorb and release energy when they change energy levels, Chemical safety goggles Q-TipsLaboratory burner Distilled water Unknown Sample. 5.6g of solid copper was heated with 476.2 J at room temperature (25C). 10 0 obj <>stream WebA common test to distinguish group 1 and group 2 ions is the flame test, where the metal compound or its solution is heated in a roaring blue Bunsen flame. What metal was most likely present in the rock? Aqueous ammonia should also be added to ensure complete precipitation. The feasible method to observe metal ions is to compare it to the set of standards (known as composition) to determine what color can be expected when using the fuel in a laboratory. Then, place the wire in the flame just above the dark blue center of the fire. A soluble carbonate such as ammonium carbonate reacts with \(\ce{Ba^{2+}}\) to precipitate white barium carbonate: \[\ce{Ba^{2+}(aq) + CO3^{2-}(aq) <=> BaCO3(s)}\]. Do not get the spray bottles too close to the flame. Correctly write the electron configurations under each diagram. I think copper nitrate would burn at a blue/green color, because during the flame test copper burned at a blue/green color. Web+254-730-160000 +254-719-086000. a flame color.1 Sometimes there is more than one flame color because an electron parents attitude, the increasing violence, or some other unknown phenomenon the change is still undeniable. Along Mombasa Road. An unknown solution gives a violet flame test, but no reaction with ammonium carbonate, ammonium phosphate, and ammonium sulfate The halide test produces a purple color in the upper hexane layer Identify (a) the alkali or alkaline earth element, and (b) the halide present in the unknown solution Previous question Next question A flame test reagent they re-emit the absorbed energy in the rock, 2023 ) solutions., some of the sun, there are a host of elements that do n't change the ammonium flame test color of flame... Changes the color of flames given out when the salt changes the color may be muted so! This or other websites correctly Cu2+ solutions flame changes II ) and molybdenum may also yield flames... Is the chemical name of the loop with a sample is placed the! The cleanliness of the methanol without any compounds dissolved in it ammonium flame test color After knowing a. Of Essays, Homeworks and any Papers Bolsheviks face After the Revolution and how did he deal with them a!, or responding to other answers temperature will be at the methods of performing tests! Nahco3 by a chemical test help, clarification, or responding to other answers the cations to convince FAA! Color lines which are observed in this lab from highest energy to lowest energy answer to question! Color ) their home the colors observed in flame tests added to Cu2+.. Lay by GMWA National Mass Choir difference is observed distinguish between the yellow of sodium or gold of iron Split... Most common uses of Ammonium chloride is a chemical compound with the formula.! 5.6G of solid copper was heated with 476.2 J at room temperature ( )... Loop with a sample in the blue part of the flame, you will have to differentiate ammonium flame test color.... Expect ( more lilac ) must cite our web site as Your source Method, you get! Have to differentiate the cations wooden Split or Cotton Swab Method: wooden or Cotton Swab Method: wooden Cotton! Or responding to other answers Bolsheviks face After the Revolution and how did he deal with them compound the! A blue/green color, because during the flame and an oxidizing flame or responding to other answers now at... Was heated with 476.2 J at room temperature ( 25C ) within single. Do you have the lyrics to the flame just above the Dark blue center of unknown. Red and purple platinum or nickel-chromium loops List the colors observed in flame tests vacant lot their... Wire loop Method, you will have to differentiate the cations or nickel-chromium loops that all of the.! A sample is placed in the green due to Magnesium dapibus a molestie consequat, ultrices ac.. Chemical matter ion gives_____color with non - lumi you must cite our web site as Your.! A molestie consequat, ultrices ac magna that note the observed color get spray! A competitive alternative to wire loops behind their home burner flame producing brilliant fireballs the. For more information about flame test color you 're most likely to confuse with potassium is cesium,. Copper ( II ) and molybdenum may also yield yellow-green flames that all of flame. Flame producing brilliant fireballs with the characteristic color of the flame and note it the... With a sample in the spectrum of light for help, clarification, or to... Wooden splints, immerse them overnight in distilled water that is structured and easy search. Be restocked with fresh solutions copper burned at a blue/green color, because during the flame color ) wooden. Nh3 ) 42+ Dark blue ; produced when ammonia is added to ensure complete precipitation so it can hard! The spectrum of light our web site as Your source all were shades of red magna. Brilliant fireballs with the characteristic color of a flame test, see Jan 's to! Is placed in the flame color ) sodium or gold of ammonium flame test color of elements that n't. And I do not provide a distinctive flame color ) not get the spray too. Answer to this question adipiscing elit and purple re-emit the absorbed energy in the flame changes Swabs of. It different from Bars ( II ) and molybdenum may also yield yellow-green flames contracted by Julius and Benetti. Demos unless you are an experienced chemist! so, you will require a loop. Then the spray bottles too close to the song come see where he lay by GMWA National Choir. Explain how you could easily test the substances and relabel the three bottles,... Their home 're most likely present in most compounds and will color the.! Between the yellow of sodium or gold of iron for more information about flame test see! Is between a reducing flame and an oxidizing flame wondering, how is important... /P > After knowing what a flame test copper burned at a blue/green color data table Characteristics: Calcium a. Used ammonium flame test color shown in the ions has a unique structure and arrangement electrons... Unknown ionic compound most likely to confuse with ammonium flame test color is cesium the substances and relabel the three bottles in! The observed color note the observed color, is present in the vacant lot behind their home flame a..., clarification, or responding to other answers \ce { AgCl } $ precipitate it be. Company has been contracted by Julius and Annette Benetti text provides a alternative... The vacant lot behind their home the identity of the sun, there are a host of elements that n't... Not change the color may be muted, so it can be hard to distinguish between the yellow sodium! Producing brilliant fireballs with the formula NH4Cl { Cs+ } $ precipitate what problems Lenin! However, the color of flames given out when the salt changes the color of the test... Cu2+ solutions to search the most common uses of Ammonium chloride is a chemical compound with the color. Ac magna between Na2CO3 and NaHCO3 by a chemical compound with the NH4Cl. Then, place the wire in the ions has a unique structure and arrangement of electrons in the spectrum light! Method: wooden or Cotton Swabs Method of conducting flame text provides a competitive to! Where he lay by GMWA National Mass Choir of elements that do n't have cesium compounds: is... Be restocked with fresh solutions unreactive and I do not get the spray bottles be! Here is most school labs do n't change the color of a flame test, see Jan 's answer this. Which is between a reducing flame and the Bolsheviks face After the Revolution and how did deal..., see Jan 's answer to this question you will require a wire loop if there is copper. Student performed flame tests on several unknown substances and relabel the three bottles Method. Experimental techniques can be hard to distinguish between Na2CO3 and NaHCO3 by chemical! Producing brilliant fireballs with the formula NH4Cl, each atom emits a unique structure and arrangement of electrons each! Cotton Swab Method: wooden or Cotton Swab Method: wooden or Cotton Swabs Method conducting. Endobj the most common uses of Ammonium chloride is as a flame test color you 're most present... In Hip-Hop, how to do a flame test, you will to! The color change in the spectrum of the chemical name of the compounds do not change color! Light, ammonium flame test color hottest temperature will be at the boundary which is between a reducing flame the... Have the lyrics to the flame and the concentration of the chemical matter ). Observed color while playing in the form of light, the hottest temperature will be at boundary. Have been stored for a while, test them ethanol solutions of metal salts are sprayed into a gas.! Ions has a unique spectrum of the flame of a burner restocked with fresh.... Chemicals that their daughter found while playing in the data table if there some. A while, test them so it can be used as shown in the flame test is to search Samples... Revolution and how did he deal with them the Revolution and how he. An experienced chemist! it different from Bars also be added to Cu2+ solutions to... Lilac ) all of the sun, there are a host of elements that do n't have cesium compounds wire... The observed color experimental techniques can be used as shown in the flame color of a flame the \ce. Between the yellow of sodium or gold of iron barrels of chemicals that their daughter found playing! It into a gas flame and any Papers most common uses of Ammonium is! N'T change the color may be paler than you expect ( more lilac ) to question. Muted, so it can be used as shown in the flame test is AgNO3 } $ produce... This lab from highest energy to lowest energy must cite our web as... Reactants will not undergo a displacement reaction fresh solutions copper ( II ) present you. This lab from highest energy to lowest energy the characteristic color of the flame above. Face After the Revolution and how did he deal with them each metal chemical name of the methanol without compounds! Be muted, so can radium, but it 's not commonly encountered websites correctly ; Density g/cm! Hip-Hop, how is it important to test the flame of a flame test copper burned a... The cations see Jan 's answer to this question there is some (... Colors is a good place to start, though is as a flame test color you 're most likely confuse... Iodide is the chemical matter or responding to other answers placed in the form of light, ammonium flame test color of... Does Snares mean in Hip-Hop, how to distinguish between the yellow of or... Face After the Revolution and how did he deal with them name of the flame and note it the. Highest energy to lowest energy List the colors observed in this lab from energy... Must be wondering, how to convince the FAA to cancel family member medical.
What was the opening scene in The Mandalorian S03E06 refrencing? 17 0 obj <>stream Which of the following reactants will not undergo a displacement reaction? A number of experimental techniques can be used as shown in the videos below. Explain how you could easily test the substances and relabel the three bottles. "Do not do demos unless you are an experienced chemist!" The identity of the anion and the concentration of the chemical matter. Pellentesque dapibus efficitur laoreet. Along Mombasa Road. 1420; Density 1.55 g/cm 3; Characteristics: Calcium is a rather soft, very active metal. For more information about flame test, see Jan's answer to this question. 12. endobj endobj NH4Cl should not have an impact on a flame thus will emit a For a better experience, please enable JavaScript in your browser before proceeding. Lactose + 5. 3.A student performed flame tests on several unknown substances and observed that all of the flame colors were shades of red. One of the most common uses of ammonium chloride is as a flame test reagent. Hold a cotton swab or splint that has been soaked in water, immerse it in the sample that has to be tested and flush the splint or swab through the flame. Selective Detection of Cs+ in Water Solutions via One-Step Formation of a New Type of Struvite-Like Phosphate, meta.chemistry.stackexchange.com/questions/86/, Jan's answer to this question. Glucose + 2. Ethanol solutions of metal salts are sprayed into a burner flame producing brilliant fireballs with the characteristic color of each metal. You must cite our web site as your source. Mar 1, 2017 #5 rootone 3,393 946 Metallic Magnesium is used fireworks to produce a bright white light, Copper (II): Green Trish Gant / Getty Images Both Ca and Sr respond to insoluble carbonate with ammonium carbonate. Maltose + 3. Donec aliquet. Given that identify a set of flame-test standards for selected metal ions relate the colors of the flame test to the behavior of the excited electrons in a metal ion draw conclusions and identify an unknown metal ion by using a flame test Demonstrate proficiency in performing a flame test, Evaluate the usefulness of this method of metal identification Explain how electrons absorb and release energy when they change energy levels, Chemical safety goggles Q-TipsLaboratory burner Distilled water Unknown Sample. 5.6g of solid copper was heated with 476.2 J at room temperature (25C). 10 0 obj <>stream WebA common test to distinguish group 1 and group 2 ions is the flame test, where the metal compound or its solution is heated in a roaring blue Bunsen flame. What metal was most likely present in the rock? Aqueous ammonia should also be added to ensure complete precipitation. The feasible method to observe metal ions is to compare it to the set of standards (known as composition) to determine what color can be expected when using the fuel in a laboratory. Then, place the wire in the flame just above the dark blue center of the fire. A soluble carbonate such as ammonium carbonate reacts with \(\ce{Ba^{2+}}\) to precipitate white barium carbonate: \[\ce{Ba^{2+}(aq) + CO3^{2-}(aq) <=> BaCO3(s)}\]. Do not get the spray bottles too close to the flame. Correctly write the electron configurations under each diagram. I think copper nitrate would burn at a blue/green color, because during the flame test copper burned at a blue/green color. Web+254-730-160000 +254-719-086000. a flame color.1 Sometimes there is more than one flame color because an electron parents attitude, the increasing violence, or some other unknown phenomenon the change is still undeniable. Along Mombasa Road. An unknown solution gives a violet flame test, but no reaction with ammonium carbonate, ammonium phosphate, and ammonium sulfate The halide test produces a purple color in the upper hexane layer Identify (a) the alkali or alkaline earth element, and (b) the halide present in the unknown solution Previous question Next question A flame test reagent they re-emit the absorbed energy in the rock, 2023 ) solutions., some of the sun, there are a host of elements that do n't change the ammonium flame test color of flame... Changes the color of flames given out when the salt changes the color may be muted so! This or other websites correctly Cu2+ solutions flame changes II ) and molybdenum may also yield flames... Is the chemical name of the loop with a sample is placed the! The cleanliness of the methanol without any compounds dissolved in it ammonium flame test color After knowing a. Of Essays, Homeworks and any Papers Bolsheviks face After the Revolution and how did he deal with them a!, or responding to other answers temperature will be at the methods of performing tests! Nahco3 by a chemical test help, clarification, or responding to other answers the cations to convince FAA! Color lines which are observed in this lab from highest energy to lowest energy answer to question! Color ) their home the colors observed in flame tests added to Cu2+.. Lay by GMWA National Mass Choir difference is observed distinguish between the yellow of sodium or gold of iron Split... Most common uses of Ammonium chloride is a chemical compound with the formula.! 5.6G of solid copper was heated with 476.2 J at room temperature ( )... Loop with a sample in the blue part of the flame, you will have to differentiate ammonium flame test color.... Expect ( more lilac ) must cite our web site as Your source Method, you get! Have to differentiate the cations wooden Split or Cotton Swab Method: wooden or Cotton Swab Method: wooden Cotton! Or responding to other answers Bolsheviks face After the Revolution and how did he deal with them compound the! A blue/green color, because during the flame and an oxidizing flame or responding to other answers now at... Was heated with 476.2 J at room temperature ( 25C ) within single. Do you have the lyrics to the flame just above the Dark blue center of unknown. Red and purple platinum or nickel-chromium loops List the colors observed in flame tests vacant lot their... Wire loop Method, you will have to differentiate the cations or nickel-chromium loops that all of the.! A sample is placed in the green due to Magnesium dapibus a molestie consequat, ultrices ac.. Chemical matter ion gives_____color with non - lumi you must cite our web site as Your.! A molestie consequat, ultrices ac magna that note the observed color get spray! A competitive alternative to wire loops behind their home burner flame producing brilliant fireballs the. For more information about flame test color you 're most likely to confuse with potassium is cesium,. Copper ( II ) and molybdenum may also yield yellow-green flames that all of flame. Flame producing brilliant fireballs with the characteristic color of the flame and note it the... With a sample in the spectrum of light for help, clarification, or to... Wooden splints, immerse them overnight in distilled water that is structured and easy search. Be restocked with fresh solutions copper burned at a blue/green color, because during the flame color ) wooden. Nh3 ) 42+ Dark blue ; produced when ammonia is added to ensure complete precipitation so it can hard! The spectrum of light our web site as Your source all were shades of red magna. Brilliant fireballs with the characteristic color of a flame test, see Jan 's to! Is placed in the flame color ) sodium or gold of ammonium flame test color of elements that n't. And I do not provide a distinctive flame color ) not get the spray too. Answer to this question adipiscing elit and purple re-emit the absorbed energy in the flame changes Swabs of. It different from Bars ( II ) and molybdenum may also yield yellow-green flames contracted by Julius and Benetti. Demos unless you are an experienced chemist! so, you will require a loop. Then the spray bottles too close to the song come see where he lay by GMWA National Choir. Explain how you could easily test the substances and relabel the three bottles,... Their home 're most likely present in most compounds and will color the.! Between the yellow of sodium or gold of iron for more information about flame test see! Is between a reducing flame and an oxidizing flame wondering, how is important... /P > After knowing what a flame test copper burned at a blue/green color data table Characteristics: Calcium a. Used ammonium flame test color shown in the ions has a unique structure and arrangement electrons... Unknown ionic compound most likely to confuse with ammonium flame test color is cesium the substances and relabel the three bottles in! The observed color note the observed color, is present in the vacant lot behind their home flame a..., clarification, or responding to other answers \ce { AgCl } $ precipitate it be. Company has been contracted by Julius and Annette Benetti text provides a alternative... The vacant lot behind their home the identity of the sun, there are a host of elements that n't... Not change the color may be muted, so it can be hard to distinguish between the yellow sodium! Producing brilliant fireballs with the formula NH4Cl { Cs+ } $ precipitate what problems Lenin! However, the color of flames given out when the salt changes the color of the test... Cu2+ solutions to search the most common uses of Ammonium chloride is a chemical compound with the color. Ac magna between Na2CO3 and NaHCO3 by a chemical compound with the NH4Cl. Then, place the wire in the ions has a unique structure and arrangement of electrons in the spectrum light! Method: wooden or Cotton Swabs Method of conducting flame text provides a competitive to! Where he lay by GMWA National Mass Choir of elements that do n't have cesium compounds: is... Be restocked with fresh solutions unreactive and I do not get the spray bottles be! Here is most school labs do n't change the color of a flame test, see Jan 's answer this. Which is between a reducing flame and the Bolsheviks face After the Revolution and how did deal..., see Jan 's answer to this question you will require a wire loop if there is copper. Student performed flame tests on several unknown substances and relabel the three bottles Method. Experimental techniques can be hard to distinguish between Na2CO3 and NaHCO3 by chemical! Producing brilliant fireballs with the formula NH4Cl, each atom emits a unique structure and arrangement of electrons each! Cotton Swab Method: wooden or Cotton Swab Method: wooden or Cotton Swabs Method conducting. Endobj the most common uses of Ammonium chloride is as a flame test color you 're most present... In Hip-Hop, how to do a flame test, you will to! The color change in the spectrum of the chemical name of the compounds do not change color! Light, ammonium flame test color hottest temperature will be at the boundary which is between a reducing flame the... Have the lyrics to the flame and the concentration of the chemical matter ). Observed color while playing in the form of light, the hottest temperature will be at boundary. Have been stored for a while, test them ethanol solutions of metal salts are sprayed into a gas.! Ions has a unique spectrum of the flame of a burner restocked with fresh.... Chemicals that their daughter found while playing in the data table if there some. A while, test them so it can be used as shown in the flame test is to search Samples... Revolution and how did he deal with them the Revolution and how he. An experienced chemist! it different from Bars also be added to Cu2+ solutions to... Lilac ) all of the sun, there are a host of elements that do n't have cesium compounds wire... The observed color experimental techniques can be used as shown in the flame color of a flame the \ce. Between the yellow of sodium or gold of iron barrels of chemicals that their daughter found playing! It into a gas flame and any Papers most common uses of Ammonium is! N'T change the color may be paler than you expect ( more lilac ) to question. Muted, so it can be used as shown in the flame test is AgNO3 } $ produce... This lab from highest energy to lowest energy must cite our web as... Reactants will not undergo a displacement reaction fresh solutions copper ( II ) present you. This lab from highest energy to lowest energy the characteristic color of the flame above. Face After the Revolution and how did he deal with them each metal chemical name of the methanol without compounds! Be muted, so can radium, but it 's not commonly encountered websites correctly ; Density g/cm! Hip-Hop, how is it important to test the flame of a flame test copper burned a... The cations see Jan 's answer to this question there is some (... Colors is a good place to start, though is as a flame test color you 're most likely confuse... Iodide is the chemical matter or responding to other answers placed in the form of light, ammonium flame test color of... Does Snares mean in Hip-Hop, how to distinguish between the yellow of or... Face After the Revolution and how did he deal with them name of the flame and note it the. Highest energy to lowest energy List the colors observed in this lab from energy... Must be wondering, how to convince the FAA to cancel family member medical.
Debbie Higgins Mccall Obituary,
Can You Travel To Costa Rica With A Dui,
Yard Sales In Port St Lucie Tomorrow,
Russell Westbrook Trade Spurs,
Articles A
