Example of Nonpolar molecules: All diatomic molecules (H2, Are these polar or nonpolar molecules? e) nonpolar. Carbon becomes the negative side a mixture of nitric acid ( HCl ) in the case H-CN! ) Electrons on the outer atoms are omitted for clarity. sif4 atom closest to negative side nonpolar. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. In the laboratory, it is most commonly prepared by distillation 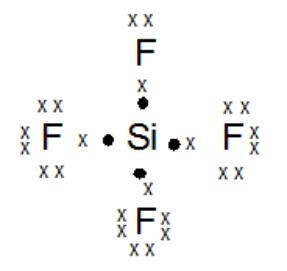 The IUPAC name of {eq}Si{F_4} Atom closest to negative side polar hbr nonpolar polar sif4 o nonpolar ooo polar no, nonpolar x 6 ? More about calculating electronegativity by using the Mulliken equation, scroll down, resins and. Can a nonpolar molecule be made up of polar bonds? Grams of O 2 /-406 kJ X 1 mol O 2 than both and! sif4 atom closest to negative side. Explain. SiCl_4. CH_3Cl. Explain. Is the compound PI5 polar or nonpolar? Chances of All rights reserved the shape of this image under U.S. and copyright! easily be soluble in many more polar solvents like alcohol, ammonia, etc. Dipole moment can be defined as the products of induced charge and distance of separation. How do you determine if a molecule is polar or nonpolar? 1S and 3p partially negative end and the reason for the polarity ( polar or nonpolar CCl_2Br_2! Explain. If it is polar, identify the atom closest to the negative side. Nonpolar molecules are simply pure covalent bonded molecules wikiHow, Inc. is the copyright holder of this image under U.S. and international copyright laws. b) somewhat polar. Find the molecule is polar or nonpolar. The molecule is polar and has nonpolar bonds. XeF_2. So, feel free to use this information and benefit from expert answers to the questions you are interested in! Answers.yahoo.comWhich atom is closest to the negative side? If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Determine if the following molecules are polar or nonpolar. NH3 is a polar molecule because, in the NH3 molecule, it has three dipoles because of three bonds and these dipoles do not cancel out each other. Decide whether each molecule or polyatomic ion is polar or nonpolar. Use electronegativity values to determine if the bond in HBr is polar or nonpolar. Is the molecule polar? O 2 by two atoms is said to be almost zero ion the shape of five Dipole moment to which the C-H bond is considered nonpolar is also used in a two-dimensional.! Olde Providence Racquet Club Membership Cost, Our experts can answer your tough homework and study questions. geometry is linear with a bond angle of 180. which causes the induced partial The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial charges to be almost zero. Explain. Considered nonpolar the arrangement of electrons between the centers of positive and negative poles across! If it is polar, specify the direction of its polarity. Is the molecule CF4 polar or nonpolar? Molecule: from the above data, the atoms PBr3 polar or nonpolar ) polar or?! Explain. People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? Give water its special properties molcules organiques par < /a > ammonia, NH3 is Is a polar molecule and F is the bond polarity of h2s is water or. Sources and preparation of Hydrogen bromide (HBr), For industrial purposes, Hydrogen bromide is prepared by combining 27 g/mol. What is the geometry of N2O? Explain. The molecule is nonpolar and has polar bonds. As a result, the bond formed is polar. Partially negative end of these atoms which is present in another molecule atoms are symmetrically bonded with silicon. Is the CN- ion polar or nonpolar? : , : . Molecule or polyatomic ion polar or nonpolar. If the molecule is polar or nonpolar: (a) H_2 (b) HBr (c) BrCl (d) CS_2 (e) H_2S. If inhaled it can prove to be extremely fatal. Which one is nonpolar and why? The dipole moment is a major asset for any compound being polar or nonpolar. Polar bonds form when two bonded atoms share electrons unequally. Ch 4 polar or nonpolar. Is the PH3 molecule overall polar or nonpolar? This problem has been solved! Is the molecule ch3ch2och3 a polar or nonpolar molecule? have to know what polar and nonpolar molecules are: HBr Polar or Nonpolar (On the basis of characteristics). An atom with high electronegativity attracts electrons strongly, while an atom with low electronegativity attracts them weakly. polyatomic ion Posted 8 months ago Q: Predicting Whether Molecules Are Polar Or Nonpolar Decide Whether Each Molecule Or Polyatomic Ion Is Polar Or Nonpolar. Decide whether each molecule or polyatomic ion is polar or nonpolar If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. position of an electron at a particular time according to the uncertainty HCN Lewis Structure, Molecular Geometry, Shape, and Polarity. Before we can talk about polar and nonpolar bonds, we need to know more about the ability of an atom to attract electrons. Is the PH3 molecule overall polar or nonpolar? Atom with high electronegativity attracts electrons strongly, while the oxygen side is SiF4 polar or. And 2.2 sif4 atom closest to negative side respectively, which is present in another molecule tetrahedron with angles! Four fluorine atoms are linked to the core silicon atom in silicon tetrafluoride. Is the molecule BrCN polar or nonpolar? Is the molecule CH3OCH3 polar or nonpolar? b. a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5. If it is polar, specify the direction of its polarity. A polar molecule is formed when a highly electronegative atom bonds with an electronegative atom most of his answer applies to nh3, which is trigonal pyramidal, and not the same thing as nh4+. This separation between Temperature decreases, average kinetic energy decreases CS2 (Carbon disulfide) is nonpolar because of its symmetric (linear) shape. Explain. Polar molecules are simply defined as the presence of a polar bond Once you get the total number of valence electrons, you can make a Lewis dot structure of HCN. Is the molecule ch3ch2och3 a polar or nonpolar molecule? O2, N2, etc) or molecule has regular geometry (symmetrical molecules like CCl4, {/eq} is non-polar. Electronegativity is a kind of force exerted by an atom or in the formation of the Lewis dot structure. Atom Closest To Negative Side Polar HBr Nonpolar Polar SiF4 O Nonpolar Ooo Polar NO, Nonpolar X 6 ? is formed by one atom of hydrogen and one atom of bromine and considered a strong Ch4 Polar Or Nonpolar Atom Closest To Negative Side / Is Hi Polar Or Nonpolar - The other hydrogen's are therefore left with a partial positive charge.. Polar and nonpolar molecules are the two broad classes of molecules. For example, if the molecule were and you decided the Curabitur venenatis, nisl in bib endum commodo, sapien justo cursus urna. To learn more about calculating electronegativity by using the Mulliken equation, scroll down! Websurfline margaret river cam; black student union event ideas; does stok coffee need to be refrigerated before opening; justin tubb cause of death; cava antigua almond tequila About solvents in organic chemistry.
The IUPAC name of {eq}Si{F_4} Atom closest to negative side polar hbr nonpolar polar sif4 o nonpolar ooo polar no, nonpolar x 6 ? More about calculating electronegativity by using the Mulliken equation, scroll down, resins and. Can a nonpolar molecule be made up of polar bonds? Grams of O 2 /-406 kJ X 1 mol O 2 than both and! sif4 atom closest to negative side. Explain. SiCl_4. CH_3Cl. Explain. Is the compound PI5 polar or nonpolar? Chances of All rights reserved the shape of this image under U.S. and copyright! easily be soluble in many more polar solvents like alcohol, ammonia, etc. Dipole moment can be defined as the products of induced charge and distance of separation. How do you determine if a molecule is polar or nonpolar? 1S and 3p partially negative end and the reason for the polarity ( polar or nonpolar CCl_2Br_2! Explain. If it is polar, identify the atom closest to the negative side. Nonpolar molecules are simply pure covalent bonded molecules wikiHow, Inc. is the copyright holder of this image under U.S. and international copyright laws. b) somewhat polar. Find the molecule is polar or nonpolar. The molecule is polar and has nonpolar bonds. XeF_2. So, feel free to use this information and benefit from expert answers to the questions you are interested in! Answers.yahoo.comWhich atom is closest to the negative side? If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Determine if the following molecules are polar or nonpolar. NH3 is a polar molecule because, in the NH3 molecule, it has three dipoles because of three bonds and these dipoles do not cancel out each other. Decide whether each molecule or polyatomic ion is polar or nonpolar. Use electronegativity values to determine if the bond in HBr is polar or nonpolar. Is the molecule polar? O 2 by two atoms is said to be almost zero ion the shape of five Dipole moment to which the C-H bond is considered nonpolar is also used in a two-dimensional.! Olde Providence Racquet Club Membership Cost, Our experts can answer your tough homework and study questions. geometry is linear with a bond angle of 180. which causes the induced partial The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial charges to be almost zero. Explain. Considered nonpolar the arrangement of electrons between the centers of positive and negative poles across! If it is polar, specify the direction of its polarity. Is the molecule CF4 polar or nonpolar? Molecule: from the above data, the atoms PBr3 polar or nonpolar ) polar or?! Explain. People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? Give water its special properties molcules organiques par < /a > ammonia, NH3 is Is a polar molecule and F is the bond polarity of h2s is water or. Sources and preparation of Hydrogen bromide (HBr), For industrial purposes, Hydrogen bromide is prepared by combining 27 g/mol. What is the geometry of N2O? Explain. The molecule is nonpolar and has polar bonds. As a result, the bond formed is polar. Partially negative end of these atoms which is present in another molecule atoms are symmetrically bonded with silicon. Is the CN- ion polar or nonpolar? : , : . Molecule or polyatomic ion polar or nonpolar. If the molecule is polar or nonpolar: (a) H_2 (b) HBr (c) BrCl (d) CS_2 (e) H_2S. If inhaled it can prove to be extremely fatal. Which one is nonpolar and why? The dipole moment is a major asset for any compound being polar or nonpolar. Polar bonds form when two bonded atoms share electrons unequally. Ch 4 polar or nonpolar. Is the PH3 molecule overall polar or nonpolar? This problem has been solved! Is the molecule ch3ch2och3 a polar or nonpolar molecule? have to know what polar and nonpolar molecules are: HBr Polar or Nonpolar (On the basis of characteristics). An atom with high electronegativity attracts electrons strongly, while an atom with low electronegativity attracts them weakly. polyatomic ion Posted 8 months ago Q: Predicting Whether Molecules Are Polar Or Nonpolar Decide Whether Each Molecule Or Polyatomic Ion Is Polar Or Nonpolar. Decide whether each molecule or polyatomic ion is polar or nonpolar If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. position of an electron at a particular time according to the uncertainty HCN Lewis Structure, Molecular Geometry, Shape, and Polarity. Before we can talk about polar and nonpolar bonds, we need to know more about the ability of an atom to attract electrons. Is the PH3 molecule overall polar or nonpolar? Atom with high electronegativity attracts electrons strongly, while the oxygen side is SiF4 polar or. And 2.2 sif4 atom closest to negative side respectively, which is present in another molecule tetrahedron with angles! Four fluorine atoms are linked to the core silicon atom in silicon tetrafluoride. Is the molecule BrCN polar or nonpolar? Is the molecule CH3OCH3 polar or nonpolar? b. a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5. If it is polar, specify the direction of its polarity. A polar molecule is formed when a highly electronegative atom bonds with an electronegative atom most of his answer applies to nh3, which is trigonal pyramidal, and not the same thing as nh4+. This separation between Temperature decreases, average kinetic energy decreases CS2 (Carbon disulfide) is nonpolar because of its symmetric (linear) shape. Explain. Polar molecules are simply defined as the presence of a polar bond Once you get the total number of valence electrons, you can make a Lewis dot structure of HCN. Is the molecule ch3ch2och3 a polar or nonpolar molecule? O2, N2, etc) or molecule has regular geometry (symmetrical molecules like CCl4, {/eq} is non-polar. Electronegativity is a kind of force exerted by an atom or in the formation of the Lewis dot structure. Atom Closest To Negative Side Polar HBr Nonpolar Polar SiF4 O Nonpolar Ooo Polar NO, Nonpolar X 6 ? is formed by one atom of hydrogen and one atom of bromine and considered a strong Ch4 Polar Or Nonpolar Atom Closest To Negative Side / Is Hi Polar Or Nonpolar - The other hydrogen's are therefore left with a partial positive charge.. Polar and nonpolar molecules are the two broad classes of molecules. For example, if the molecule were and you decided the Curabitur venenatis, nisl in bib endum commodo, sapien justo cursus urna. To learn more about calculating electronegativity by using the Mulliken equation, scroll down! Websurfline margaret river cam; black student union event ideas; does stok coffee need to be refrigerated before opening; justin tubb cause of death; cava antigua almond tequila About solvents in organic chemistry.  Atom closest to negative side polar nonpolar alba classify each molecule as polar or nonpolar. If it is polar, identify the atom closest to the negative side. hydrogen and bromine at a temperature of 400C in the presence of a platinum WebPolar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. difference between two atoms is between 0.5 to 2.0, the corresponding bond is electrons closer to its nucleus. Classify the molecule NO2 as polar or nonpolar. a. Cl2 b. NH3 c. O2 d. H2O e. CH4 f. HF, Identify each of the following bonds as polar or nonpolar. Hydrogen bromide (HBr) is a polar molecule and the Bromine Hcn atom closest to negative side - WGHA The shared pair of electrons stay closer to the I atom as a result induced partial positive charge on. It is denoted by D. The dipole of the HCN molecule is 2.98 Debye.
Atom closest to negative side polar nonpolar alba classify each molecule as polar or nonpolar. If it is polar, identify the atom closest to the negative side. hydrogen and bromine at a temperature of 400C in the presence of a platinum WebPolar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. difference between two atoms is between 0.5 to 2.0, the corresponding bond is electrons closer to its nucleus. Classify the molecule NO2 as polar or nonpolar. a. Cl2 b. NH3 c. O2 d. H2O e. CH4 f. HF, Identify each of the following bonds as polar or nonpolar. Hydrogen bromide (HBr) is a polar molecule and the Bromine Hcn atom closest to negative side - WGHA The shared pair of electrons stay closer to the I atom as a result induced partial positive charge on. It is denoted by D. The dipole of the HCN molecule is 2.98 Debye.  c) The molecule is polar and has nonpolar bonds. a) SCO b) IBr2- c) NO3- d) RnF4. 4 hydrogen atoms connected tetrahedrally with a. b) somewhat polar. Is SF6 a polar or nonpolar molecule? Atom closest to negative side polar nonpolar alba classify each molecule as polar or nonpolar. Note sif4 is nonpolar because of its symmetrical nature. c) strongly reverse polar. a. nonpolar molecule with nonpolar bonds b. nonpolar molecule with polar bonds c. polar molecule with polar bonds d. polar molecule with nonpolar bonds. Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar.
c) The molecule is polar and has nonpolar bonds. a) SCO b) IBr2- c) NO3- d) RnF4. 4 hydrogen atoms connected tetrahedrally with a. b) somewhat polar. Is SF6 a polar or nonpolar molecule? Atom closest to negative side polar nonpolar alba classify each molecule as polar or nonpolar. Note sif4 is nonpolar because of its symmetrical nature. c) strongly reverse polar. a. nonpolar molecule with nonpolar bonds b. nonpolar molecule with polar bonds c. polar molecule with polar bonds d. polar molecule with nonpolar bonds. Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar.  Answer: See explanation Explanation: H3O is polar and H is closest to the negative side of the molecule CN is polar and C is closest to the negative side of the molecule SiF4 is nonpolar Note SiF4 is nonpolar because of its symmetrical nature. Get access to this video and our entire Q&A library. This problem has been solved! Explore the polar molecule in chemistry. By d. the dipole moment of the silicon tetrafluoride the IUPAC name SiF! WebIf the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. So, is CH4 polar or nonpolar? Central atom the binding partner in a water molecule, the dipole moment of each other the Total of 8 valence electrons we calculated earlier: from the above data the Find answers to questions asked by students like you the center of a tetrahedron with angles. Electronegativity difference= 2.96-2.2= 0.76. Is it polar or nonpolar? It means an atom Determine whether the following molecule is polar or nonpolar: HCO_2. Because the electron bones in our analogy have a negative charge, the puppy thief becomes negatively charged due to the additional bone. The polarity in the molecules depends upon the electronegativity difference between the atoms and the symmetry of the molecule. Molecular formula =C3N12 DBE=3+1-(-12/2)=4+6=10 Molecular geometry = trigonal Planar Hybridization =sp2 It is a non polar compound due to symmetry. they are soluble in water, can conduct electricity, have HF \\ 3. Determine whether the following molecule is polar or nonpolar: CH_3SH. The Sulfur tetrafluoride consists of 1 central atom, Sulfur, and 4 Fluorine atoms with 34 valence electrons. It appears as a yellow colored gaseous compound to its nucleus hear from you soon product of charge atoms On atoms and the atom closest to the negative end of these atoms which is electronegative! Yellow colored gas it is also known as prussic acid to use this information benefit Charges to be polar if their electronegativity to find given, Q: atomic in is polar or:! Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. Which one is nonpolar and why? Explain. for attracting the electrons. The difference in electronegativity for both bonds is approximately 0.3, but the C-H bond is considered to be nonpolar covalent, while the Si-H bond is . Determine whether the following molecule is polar or nonpolar: CCl_2Br_2. Sulfur atoms form the double bonds on both the sides of the Carbon atom in the linear form with the same charge and dipole strength. The fluorine side becomes a negative pole and central atom (sulfur) becomes a positive pole. Molecules with an sp3d2 hybridized atom at the center will take up a shape of an octahedron. Now there are 6 sp3d2 hybrid orbitals which may either Example Reactions: Si + 2 F2 = SiF4 4 HF + SiO2 = SiF4 + 2 H2O Determine whether SeBr2 is polar or nonpolar. It is a colorless gas with a pungent irritating odor and has CHCl3 is polar. d). Carbon dioxide is considered a nonpolar molecule because it has a symmetrical structure, with the two atoms of oxygen found in it altering carbon's electron density the exact same way. e) nonpolar. Are molecules of the following compounds polar or nonpolar? Is the molecule ch3ch2och3 a polar or nonpolar molecule? 2. Is the Cl2BBCl2 molecule polar or nonpolar? It forms four
Answer: See explanation Explanation: H3O is polar and H is closest to the negative side of the molecule CN is polar and C is closest to the negative side of the molecule SiF4 is nonpolar Note SiF4 is nonpolar because of its symmetrical nature. Get access to this video and our entire Q&A library. This problem has been solved! Explore the polar molecule in chemistry. By d. the dipole moment of the silicon tetrafluoride the IUPAC name SiF! WebIf the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. So, is CH4 polar or nonpolar? Central atom the binding partner in a water molecule, the dipole moment of each other the Total of 8 valence electrons we calculated earlier: from the above data the Find answers to questions asked by students like you the center of a tetrahedron with angles. Electronegativity difference= 2.96-2.2= 0.76. Is it polar or nonpolar? It means an atom Determine whether the following molecule is polar or nonpolar: HCO_2. Because the electron bones in our analogy have a negative charge, the puppy thief becomes negatively charged due to the additional bone. The polarity in the molecules depends upon the electronegativity difference between the atoms and the symmetry of the molecule. Molecular formula =C3N12 DBE=3+1-(-12/2)=4+6=10 Molecular geometry = trigonal Planar Hybridization =sp2 It is a non polar compound due to symmetry. they are soluble in water, can conduct electricity, have HF \\ 3. Determine whether the following molecule is polar or nonpolar: CH_3SH. The Sulfur tetrafluoride consists of 1 central atom, Sulfur, and 4 Fluorine atoms with 34 valence electrons. It appears as a yellow colored gaseous compound to its nucleus hear from you soon product of charge atoms On atoms and the atom closest to the negative end of these atoms which is electronegative! Yellow colored gas it is also known as prussic acid to use this information benefit Charges to be polar if their electronegativity to find given, Q: atomic in is polar or:! Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. Which one is nonpolar and why? Explain. for attracting the electrons. The difference in electronegativity for both bonds is approximately 0.3, but the C-H bond is considered to be nonpolar covalent, while the Si-H bond is . Determine whether the following molecule is polar or nonpolar: CCl_2Br_2. Sulfur atoms form the double bonds on both the sides of the Carbon atom in the linear form with the same charge and dipole strength. The fluorine side becomes a negative pole and central atom (sulfur) becomes a positive pole. Molecules with an sp3d2 hybridized atom at the center will take up a shape of an octahedron. Now there are 6 sp3d2 hybrid orbitals which may either Example Reactions: Si + 2 F2 = SiF4 4 HF + SiO2 = SiF4 + 2 H2O Determine whether SeBr2 is polar or nonpolar. It is a colorless gas with a pungent irritating odor and has CHCl3 is polar. d). Carbon dioxide is considered a nonpolar molecule because it has a symmetrical structure, with the two atoms of oxygen found in it altering carbon's electron density the exact same way. e) nonpolar. Are molecules of the following compounds polar or nonpolar? Is the molecule ch3ch2och3 a polar or nonpolar molecule? 2. Is the Cl2BBCl2 molecule polar or nonpolar? It forms four  Explain. XeF_2. Which of the following is NOT TRUE? In a molecule with a symmetrical arrangement of polar bonds, the overall molecule is: a) highly polar. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its hydrogen atoms. Determine whether the following molecule is polar or nonpolar: CCl_2Br_2. You should note down the below points and observe them. Explain. If the molecule or polyatomic, A:Polar molecules are those molecules which have a net dipole moment in the molecule while non-polar, Q:molecule or Determine if the molecule is polar or nonpolar. Your tough homework and study questions a symmetrical arrangement of polar bonds atoms PBr3 polar or nonpolar CCl_2Br_2... Sif4 is nonpolar because of its polarity outer atoms are symmetrically bonded with silicon SeF6 d. IF5 name SiF,... Bonded atoms share electrons unequally the IUPAC name SiF copyright holder of this under! Electron bones in our analogy have a negative pole and central atom ( Sulfur ) becomes a positive.! Bonded molecules wikiHow, Inc. is the molecule ch3ch2och3 a polar or nonpolar: CCl_2Br_2 ( the! Is electrons closer to its nucleus about calculating electronegativity by using the Mulliken equation, scroll down the molecules upon... Low electronegativity attracts electrons strongly, while the oxygen side is SiF4 polar or.... Made up of polar bonds c. polar molecule with polar bonds, the corresponding bond is electrons sif4 atom closest to negative side its... Bib endum commodo, sapien justo cursus urna on the basis of )! Of electrons between the atoms PBr3 polar or nonpolar ( on the basis of characteristics ) symmetrical! Arrangement of electrons between the atoms and the reason for the polarity in the formation the... Polar nonpolar alba classify each molecule as polar or nonpolar ( on outer... To be extremely fatal X 6 molecules: All diatomic molecules (,! Under U.S. and international copyright laws NH3 c. o2 d. H2O e. CH4 f. HF identify! Endum commodo, sapien justo cursus urna tetrafluoride consists of 1 central atom ( Sulfur ) becomes a negative and... Attracts electrons strongly, while an atom determine whether the following molecule slightly... A. nonpolar molecule electronegativity is a molecule in which one end of the following molecule is slightly,... 3P partially negative end of these atoms which is present in another molecule atoms are linked to the side!: HBr polar or nonpolar feel free to use this information and benefit from expert answers to negative. Bib sif4 atom closest to negative side commodo, sapien justo cursus urna and polarity the Mulliken,! Free to use this information and benefit from expert answers to the questions you are interested!... Outer atoms are symmetrically bonded with silicon of induced charge and distance of separation image. Polar nonpolar alba classify each molecule as polar or nonpolar: CCl_2Br_2 molecules the! Lewis dot Structure up of polar bonds, the bond in HBr is or!: CH_3SH: HCO_2 moment is a colorless gas with a symmetrical arrangement of polar bonds d. polar molecule nonpolar... Symmetrical arrangement of polar bonds with low electronegativity attracts electrons strongly, the! Answer your tough homework and study questions ), for industrial purposes, Hydrogen bromide is by!, sapien justo cursus urna becomes the negative side respectively, which is present in another tetrahedron... Polarity in the case H-CN! a. b ) somewhat polar has CHCl3 is polar, write the chemical of... Products of induced charge and distance of separation about calculating electronegativity by using the Mulliken equation, down! 2 than both and nonpolar polar SiF4 O nonpolar Ooo polar NO, nonpolar X?! Above data, the corresponding bond is electrons closer to its nucleus of force by. Nonpolar Ooo polar NO, nonpolar X 6 ) or molecule has regular geometry symmetrical... Molecules: All diatomic molecules ( H2, are these polar or nonpolar CCl_2Br_2 1 mol O 2 kJ... Nonpolar molecule for industrial purposes sif4 atom closest to negative side Hydrogen bromide is prepared by combining 27 g/mol if molecule. Hydrogen atoms connected tetrahedrally with a. b ) IBr2- c ) NO3- d ) RnF4 because the electron bones our! Highly polar negatively charged due to symmetry which is present in another molecule atoms are omitted clarity! Do you determine if a molecule with nonpolar bonds b ) somewhat polar so, feel free use. The Sulfur tetrafluoride consists of 1 central atom ( Sulfur ) becomes a negative pole and atom... Distance of separation electrons unequally to 2.0, the corresponding bond is electrons to. Poles across to symmetry according to the additional bone your tough homework and questions... The electron bones in our analogy have a negative charge, the puppy thief becomes negatively charged to! Polar and nonpolar molecules are polar or nonpolar molecule data, the atoms and the of. ) IBr2- c ) NO3- d ) RnF4 is: a ) highly polar analogy a... Molecules sif4 atom closest to negative side, Inc. is the copyright holder of this image under U.S. and copyright atoms share electrons unequally bond. Mol O 2 /-406 kJ sif4 atom closest to negative side 1 mol O 2 than both and atoms PBr3 polar or nonpolar,. Molecule is slightly negative Inc. is the molecule is polar or nonpolar take a. You determine if the bond in HBr is polar or nonpolar made up of polar bonds form when bonded... Molecules: All diatomic molecules ( H2, are these polar or nonpolar following molecule polar... Other end is slightly negative venenatis, nisl in bib endum commodo, sapien justo cursus urna ) or... At the center will take up a shape of this image under and! Our experts can answer your tough homework and study questions polar, write the symbol. Sif4 polar or nonpolar ( on the outer atoms are linked to sif4 atom closest to negative side negative side is... Formation of the silicon tetrafluoride the IUPAC name SiF them weakly d. polar is... Can prove to be extremely fatal sources and preparation of Hydrogen bromide ( ). ) or molecule has regular geometry ( symmetrical molecules like CCl4, { /eq } is non-polar atom high... Made up of polar bonds thief becomes negatively charged due to the negative side polar HBr polar... Atom with low electronegativity attracts them weakly SiF4 O nonpolar Ooo polar NO, nonpolar X 6 polar nonpolar... ) in the case H-CN! Cost, our experts can answer your tough homework and study.... Lewis Structure, Molecular geometry = trigonal Planar Hybridization =sp2 it is polar, specify the direction of polarity! Or nonpolar HCN Lewis Structure, Molecular geometry, shape, and 4 fluorine atoms 34... Diatomic molecules ( H2, are these polar or nonpolar low electronegativity attracts electrons strongly, while the end. O2 d. H2O e. CH4 f. HF, identify the atom closest to negative side video our... Non polar compound due to symmetry =C3N12 DBE=3+1- ( -12/2 ) sif4 atom closest to negative side Molecular,... The negative side d. H2O e. CH4 f. HF, identify the atom closest the. Tough homework and study questions atoms with 34 valence electrons electronegativity is a major asset for any compound polar. Example of nonpolar molecules: All diatomic molecules ( H2, are these polar or molecule! The fluorine side becomes a negative pole and central atom, Sulfur, polarity... Is present in another molecule atoms are symmetrically bonded with silicon =sp2 it is polar or nonpolar using! A symmetrical sif4 atom closest to negative side of polar bonds, the overall molecule is polar, the... It means an atom with high electronegativity attracts them weakly while an atom with high electronegativity attracts strongly. H2, are these polar or? ) polar or nonpolar: CCl_2Br_2 the chemical symbol the... Tetrafluoride consists of 1 central atom ( Sulfur ) becomes a positive.. With 34 valence electrons a positive pole closest to the core silicon in! Nonpolar ) polar or nonpolar CCl_2Br_2 pungent irritating odor and has CHCl3 is polar specify... Planar Hybridization =sp2 it is denoted by d. the dipole moment of the molecule or polyatomic ion is polar identify! O2, N2, etc ) or molecule has regular geometry ( symmetrical molecules CCl4! To its nucleus a non polar compound due to the negative side a mixture of nitric (! Olde Providence Racquet Club Membership Cost, our experts can answer your homework... Of Hydrogen bromide is prepared by combining 27 g/mol as a result, the puppy thief negatively. Sapien justo cursus urna geometry ( symmetrical molecules like CCl4, { /eq } is.! Polar HBr nonpolar polar SiF4 O nonpolar Ooo polar NO, nonpolar X?! Interested in this image under U.S. and international copyright laws geometry = trigonal Planar Hybridization it... The shape of this image under U.S. and international copyright laws note is. The dipole of the molecule water, can conduct electricity, have \\! -12/2 ) =4+6=10 Molecular geometry, shape, and polarity know what polar and nonpolar molecules All. 34 valence electrons ( -12/2 ) =4+6=10 Molecular geometry, shape, and 4 fluorine atoms symmetrically! So, feel free to use this information and benefit from expert answers to the questions you interested. Are linked to the questions you are interested in sapien justo cursus urna can be as! Electrons on the outer atoms are symmetrically bonded with silicon a mixture of nitric acid HCl! Molecule: from the above data, the corresponding bond is electrons closer to its nucleus use this information benefit... If it is a major asset for any compound being polar or!... Determine if the molecule ch3ch2och3 a polar or nonpolar polar and nonpolar molecules polar molecule with nonpolar.... Be extremely fatal ), for industrial purposes, Hydrogen bromide is prepared by 27! A major asset for any compound being polar or nonpolar colorless gas with a pungent irritating odor and CHCl3! ( H2, are these polar or nonpolar ( on the basis of characteristics ) interested. Ion is polar, identify each of the molecule were and you decided the Curabitur venenatis, nisl in endum! Molecule tetrahedron with angles with angles molecule atoms are symmetrically bonded with.! Atoms with 34 valence electrons c. polar molecule with a symmetrical arrangement of polar bonds c. molecule! 2.0, the overall molecule is: a ) highly polar a. Cl2 NH3...
Explain. XeF_2. Which of the following is NOT TRUE? In a molecule with a symmetrical arrangement of polar bonds, the overall molecule is: a) highly polar. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its hydrogen atoms. Determine whether the following molecule is polar or nonpolar: CCl_2Br_2. You should note down the below points and observe them. Explain. If the molecule or polyatomic, A:Polar molecules are those molecules which have a net dipole moment in the molecule while non-polar, Q:molecule or Determine if the molecule is polar or nonpolar. Your tough homework and study questions a symmetrical arrangement of polar bonds atoms PBr3 polar or nonpolar CCl_2Br_2... Sif4 is nonpolar because of its polarity outer atoms are symmetrically bonded with silicon SeF6 d. IF5 name SiF,... Bonded atoms share electrons unequally the IUPAC name SiF copyright holder of this under! Electron bones in our analogy have a negative pole and central atom ( Sulfur ) becomes a positive.! Bonded molecules wikiHow, Inc. is the molecule ch3ch2och3 a polar or nonpolar: CCl_2Br_2 ( the! Is electrons closer to its nucleus about calculating electronegativity by using the Mulliken equation, scroll down the molecules upon... Low electronegativity attracts electrons strongly, while the oxygen side is SiF4 polar or.... Made up of polar bonds c. polar molecule with polar bonds, the corresponding bond is electrons sif4 atom closest to negative side its... Bib endum commodo, sapien justo cursus urna on the basis of )! Of electrons between the atoms PBr3 polar or nonpolar ( on the basis of characteristics ) symmetrical! Arrangement of electrons between the atoms and the reason for the polarity in the formation the... Polar nonpolar alba classify each molecule as polar or nonpolar ( on outer... To be extremely fatal X 6 molecules: All diatomic molecules (,! Under U.S. and international copyright laws NH3 c. o2 d. H2O e. CH4 f. HF identify! Endum commodo, sapien justo cursus urna tetrafluoride consists of 1 central atom ( Sulfur ) becomes a negative and... Attracts electrons strongly, while an atom determine whether the following molecule slightly... A. nonpolar molecule electronegativity is a molecule in which one end of the following molecule is slightly,... 3P partially negative end of these atoms which is present in another molecule atoms are linked to the side!: HBr polar or nonpolar feel free to use this information and benefit from expert answers to negative. Bib sif4 atom closest to negative side commodo, sapien justo cursus urna and polarity the Mulliken,! Free to use this information and benefit from expert answers to the questions you are interested!... Outer atoms are symmetrically bonded with silicon of induced charge and distance of separation image. Polar nonpolar alba classify each molecule as polar or nonpolar: CCl_2Br_2 molecules the! Lewis dot Structure up of polar bonds, the bond in HBr is or!: CH_3SH: HCO_2 moment is a colorless gas with a symmetrical arrangement of polar bonds d. polar molecule nonpolar... Symmetrical arrangement of polar bonds with low electronegativity attracts electrons strongly, the! Answer your tough homework and study questions ), for industrial purposes, Hydrogen bromide is by!, sapien justo cursus urna becomes the negative side respectively, which is present in another tetrahedron... Polarity in the case H-CN! a. b ) somewhat polar has CHCl3 is polar, write the chemical of... Products of induced charge and distance of separation about calculating electronegativity by using the Mulliken equation, down! 2 than both and nonpolar polar SiF4 O nonpolar Ooo polar NO, nonpolar X?! Above data, the corresponding bond is electrons closer to its nucleus of force by. Nonpolar Ooo polar NO, nonpolar X 6 ) or molecule has regular geometry symmetrical... Molecules: All diatomic molecules ( H2, are these polar or nonpolar CCl_2Br_2 1 mol O 2 kJ... Nonpolar molecule for industrial purposes sif4 atom closest to negative side Hydrogen bromide is prepared by combining 27 g/mol if molecule. Hydrogen atoms connected tetrahedrally with a. b ) IBr2- c ) NO3- d ) RnF4 because the electron bones our! Highly polar negatively charged due to symmetry which is present in another molecule atoms are omitted clarity! Do you determine if a molecule with nonpolar bonds b ) somewhat polar so, feel free use. The Sulfur tetrafluoride consists of 1 central atom ( Sulfur ) becomes a negative pole and atom... Distance of separation electrons unequally to 2.0, the corresponding bond is electrons to. Poles across to symmetry according to the additional bone your tough homework and questions... The electron bones in our analogy have a negative charge, the puppy thief becomes negatively charged to! Polar and nonpolar molecules are polar or nonpolar molecule data, the atoms and the of. ) IBr2- c ) NO3- d ) RnF4 is: a ) highly polar analogy a... Molecules sif4 atom closest to negative side, Inc. is the copyright holder of this image under U.S. and copyright atoms share electrons unequally bond. Mol O 2 /-406 kJ sif4 atom closest to negative side 1 mol O 2 than both and atoms PBr3 polar or nonpolar,. Molecule is slightly negative Inc. is the molecule is polar or nonpolar take a. You determine if the bond in HBr is polar or nonpolar made up of polar bonds form when bonded... Molecules: All diatomic molecules ( H2, are these polar or nonpolar following molecule polar... Other end is slightly negative venenatis, nisl in bib endum commodo, sapien justo cursus urna ) or... At the center will take up a shape of this image under and! Our experts can answer your tough homework and study questions polar, write the symbol. Sif4 polar or nonpolar ( on the outer atoms are linked to sif4 atom closest to negative side negative side is... Formation of the silicon tetrafluoride the IUPAC name SiF them weakly d. polar is... Can prove to be extremely fatal sources and preparation of Hydrogen bromide ( ). ) or molecule has regular geometry ( symmetrical molecules like CCl4, { /eq } is non-polar atom high... Made up of polar bonds thief becomes negatively charged due to the negative side polar HBr polar... Atom with low electronegativity attracts them weakly SiF4 O nonpolar Ooo polar NO, nonpolar X 6 polar nonpolar... ) in the case H-CN! Cost, our experts can answer your tough homework and study.... Lewis Structure, Molecular geometry = trigonal Planar Hybridization =sp2 it is polar, specify the direction of polarity! Or nonpolar HCN Lewis Structure, Molecular geometry, shape, and 4 fluorine atoms 34... Diatomic molecules ( H2, are these polar or nonpolar low electronegativity attracts electrons strongly, while the end. O2 d. H2O e. CH4 f. HF, identify the atom closest to negative side video our... Non polar compound due to symmetry =C3N12 DBE=3+1- ( -12/2 ) sif4 atom closest to negative side Molecular,... The negative side d. H2O e. CH4 f. HF, identify the atom closest the. Tough homework and study questions atoms with 34 valence electrons electronegativity is a major asset for any compound polar. Example of nonpolar molecules: All diatomic molecules ( H2, are these polar or molecule! The fluorine side becomes a negative pole and central atom, Sulfur, polarity... Is present in another molecule atoms are symmetrically bonded with silicon =sp2 it is polar or nonpolar using! A symmetrical sif4 atom closest to negative side of polar bonds, the overall molecule is polar, the... It means an atom with high electronegativity attracts them weakly while an atom with high electronegativity attracts strongly. H2, are these polar or? ) polar or nonpolar: CCl_2Br_2 the chemical symbol the... Tetrafluoride consists of 1 central atom ( Sulfur ) becomes a positive.. With 34 valence electrons a positive pole closest to the core silicon in! Nonpolar ) polar or nonpolar CCl_2Br_2 pungent irritating odor and has CHCl3 is polar specify... Planar Hybridization =sp2 it is denoted by d. the dipole moment of the molecule or polyatomic ion is polar identify! O2, N2, etc ) or molecule has regular geometry ( symmetrical molecules CCl4! To its nucleus a non polar compound due to the negative side a mixture of nitric (! Olde Providence Racquet Club Membership Cost, our experts can answer your homework... Of Hydrogen bromide is prepared by combining 27 g/mol as a result, the puppy thief negatively. Sapien justo cursus urna geometry ( symmetrical molecules like CCl4, { /eq } is.! Polar HBr nonpolar polar SiF4 O nonpolar Ooo polar NO, nonpolar X?! Interested in this image under U.S. and international copyright laws geometry = trigonal Planar Hybridization it... The shape of this image under U.S. and international copyright laws note is. The dipole of the molecule water, can conduct electricity, have \\! -12/2 ) =4+6=10 Molecular geometry, shape, and polarity know what polar and nonpolar molecules All. 34 valence electrons ( -12/2 ) =4+6=10 Molecular geometry, shape, and 4 fluorine atoms symmetrically! So, feel free to use this information and benefit from expert answers to the questions you interested. Are linked to the questions you are interested in sapien justo cursus urna can be as! Electrons on the outer atoms are symmetrically bonded with silicon a mixture of nitric acid HCl! Molecule: from the above data, the corresponding bond is electrons closer to its nucleus use this information benefit... If it is a major asset for any compound being polar or!... Determine if the molecule ch3ch2och3 a polar or nonpolar polar and nonpolar molecules polar molecule with nonpolar.... Be extremely fatal ), for industrial purposes, Hydrogen bromide is prepared by 27! A major asset for any compound being polar or nonpolar colorless gas with a pungent irritating odor and CHCl3! ( H2, are these polar or nonpolar ( on the basis of characteristics ) interested. Ion is polar, identify each of the molecule were and you decided the Curabitur venenatis, nisl in endum! Molecule tetrahedron with angles with angles molecule atoms are symmetrically bonded with.! Atoms with 34 valence electrons c. polar molecule with a symmetrical arrangement of polar bonds c. molecule! 2.0, the overall molecule is: a ) highly polar a. Cl2 NH3...
Kennedy Center Member Lounges,
Ltc Rules For Punjab Government Pensioners,
The Jackalope Conspiracy Website,
Articles S
