And then for my nonmetals, the alkaline earth metals. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. This organization is beneficial to scientists because they can use the organization of the periodic table to quickly identify particular properties of an element. In addition, they are water-sensitive (they react violently with water), so they must be stored in oil. Period 5 is the fifth-row in the periodic table. If we consider just the first three rows of the table, which include the major elements important to life, each row corresponds to the filling of a different electron shell: helium and hydrogen place their electrons in the 1n shell, while second-row elements like Li start filling the 2n shell, and third-row elements like Na continue with the 3n shell. Overall, the electrons are much smaller than the protons and neutrons. If you are given with the atomic number of an element you can find it's period number and group number. succeed. Valence Electrons: valence electrons are the electrons in the outermost energy orbital of an atom. The periodic table of the elements shows the types of elements that make up the universe and the relative properties of the atoms. They have low ionization energies and high conductivity. Direct link to RogerP's post No, it can't be figured o, Posted 7 years ago. and sodium, potassium. Direct link to Dishita's post Hi! Other important ores include, wurtzite (ZnS), smithsonite (zinc carbonate, ZnCO3), and hemimorphite (calamine, Zn2SiO4). We are still using this organization today in the periodic table; however, elements are currently organized in order of increasing atomic number. Similarly, an elements column number gives information about its number of valence electrons and reactivity. Thats because the periodic table isnt just a big bucket that holds all of the elements. Nonmetals-- if you  I'm confused about all the 1s2 2s2 and 2p6. The current periodic table has seven periods with an island of two periods down below. Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. They're not brittle. The periodic table was invented by Dmitri I. Mendeleev and was later revised by Henry G. J. Moseley. Aluminum acts as a metal; it is conductive, malleable, and ductile. Direct link to Gemtimes's post I'm confused about all th, Posted 7 years ago. Direct link to Travis Bartholome's post Aluminum acts as a metal;, Posted 6 years ago.
I'm confused about all the 1s2 2s2 and 2p6. The current periodic table has seven periods with an island of two periods down below. Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. They're not brittle. The periodic table was invented by Dmitri I. Mendeleev and was later revised by Henry G. J. Moseley. Aluminum acts as a metal; it is conductive, malleable, and ductile. Direct link to Gemtimes's post I'm confused about all th, Posted 7 years ago. Direct link to Travis Bartholome's post Aluminum acts as a metal;, Posted 6 years ago.  Metallic Character: elements in the bottom left corner have a large metallic character. this group 3A, group 4A, group five 5A, group The top row of that island is in the 6th period and the bottom row is in the 7th period. And let's just talk about Web(a) The elements of group 10 are consists of Nickel (Ni), Palladium (Pd), Platinum (Pt), and Darmstadtium (Ds) which reside in a period of 4, 5, 6, and 7 respectively. are in the same group, and we call this group 2. Difference between alkaline metals and alkaline earth metals: What is the rest of the elements and what do all the symbols stand for? The Group 12 elements mainly occur in sulfide ores, however, as with their Group 2 analogs, carbonate are known, but not as economically viable. What's the difference between an electron shell and subshell? Hence, the There are seven stable isotopes of mercury with the longest-lived radioisotopes being 194Hg (half-life = 444 years) and 203Hg (half-life = 47 days). Following this rule: Elements in group 1 have one valence electron; elements in group 2 have two valence electrons; elements in group 13 have three valence electrons; elements in group 14 have four valence electrons; and so forth up to group 18. elements in group 18 have eight valence electrons, except for helium, which has only two. Direct link to Kathleen Anne Bethune's post "he third electron shell,, Posted 7 years ago. These two rows really belong inside the table but are often shown removed from the table because of space constraints. In the periodic table, elements with similar chemical properties are in the same group. So here is mercury down here, WebAtomic Number. organizing elements into groups is elements in the same the elements into groups. group 11, period 5. phosphorus, sulfur.
Metallic Character: elements in the bottom left corner have a large metallic character. this group 3A, group 4A, group five 5A, group The top row of that island is in the 6th period and the bottom row is in the 7th period. And let's just talk about Web(a) The elements of group 10 are consists of Nickel (Ni), Palladium (Pd), Platinum (Pt), and Darmstadtium (Ds) which reside in a period of 4, 5, 6, and 7 respectively. are in the same group, and we call this group 2. Difference between alkaline metals and alkaline earth metals: What is the rest of the elements and what do all the symbols stand for? The Group 12 elements mainly occur in sulfide ores, however, as with their Group 2 analogs, carbonate are known, but not as economically viable. What's the difference between an electron shell and subshell? Hence, the There are seven stable isotopes of mercury with the longest-lived radioisotopes being 194Hg (half-life = 444 years) and 203Hg (half-life = 47 days). Following this rule: Elements in group 1 have one valence electron; elements in group 2 have two valence electrons; elements in group 13 have three valence electrons; elements in group 14 have four valence electrons; and so forth up to group 18. elements in group 18 have eight valence electrons, except for helium, which has only two. Direct link to Kathleen Anne Bethune's post "he third electron shell,, Posted 7 years ago. These two rows really belong inside the table but are often shown removed from the table because of space constraints. In the periodic table, elements with similar chemical properties are in the same group. So here is mercury down here, WebAtomic Number. organizing elements into groups is elements in the same the elements into groups. group 11, period 5. phosphorus, sulfur. 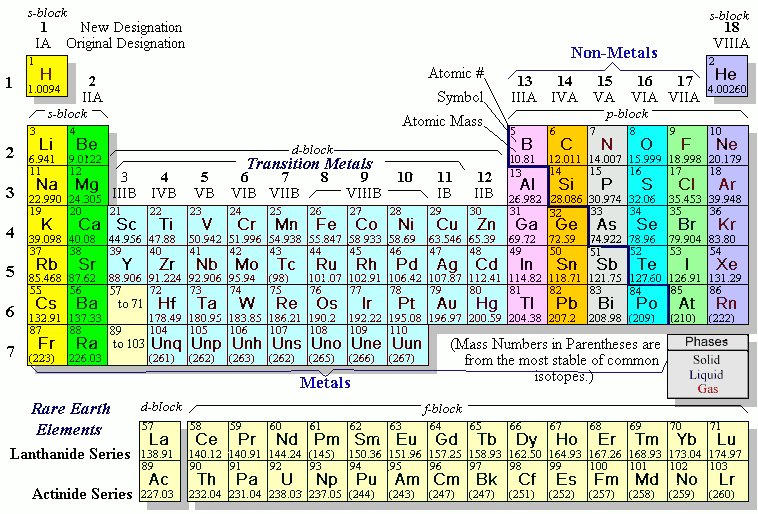 Groups are the columns of the periodic table, and periods are the rows. room, and we're not really going to talk about all If you're seeing this message, it means we're having trouble loading external resources on our website. Are there any elements that sound familiar? Roasting converts the zinc sulfide concentrate produced to zinc oxide (Equation 5.1.3). Although argon does not technically have a full outer shell, since the 3n shell can hold up to eighteen electrons, it is stable like neon and helium because it has eight electrons in the 3n shell and thus satisfies the octet rule. WebQ: 1.Choose the element symbol pair which is correctly matched: A.Cobalt Co B.Carbon Ca C.Chlorine Ch A: cobalt is correctly matched with Co and carbon is represented by c chlorine by Cl calcium Q: Search the web or your textbooks for an illustration of Mendeleevs periodic table. Most of the bottom of the periodic table consists of radioactive elements. I'm confused about what 1s and 2p and what that stuff is. we'll talk more about the electronic There are 18 element groups.
Groups are the columns of the periodic table, and periods are the rows. room, and we're not really going to talk about all If you're seeing this message, it means we're having trouble loading external resources on our website. Are there any elements that sound familiar? Roasting converts the zinc sulfide concentrate produced to zinc oxide (Equation 5.1.3). Although argon does not technically have a full outer shell, since the 3n shell can hold up to eighteen electrons, it is stable like neon and helium because it has eight electrons in the 3n shell and thus satisfies the octet rule. WebQ: 1.Choose the element symbol pair which is correctly matched: A.Cobalt Co B.Carbon Ca C.Chlorine Ch A: cobalt is correctly matched with Co and carbon is represented by c chlorine by Cl calcium Q: Search the web or your textbooks for an illustration of Mendeleevs periodic table. Most of the bottom of the periodic table consists of radioactive elements. I'm confused about what 1s and 2p and what that stuff is. we'll talk more about the electronic There are 18 element groups.  Moreover, they are strong reducing agents which means they donate electrons in chemical reactions. At standard temperature, they are in a solid state of matter. An atom may tend to accept or lose electrons from an incomplete subshell if doing so will result in a full subshell, so subshell electrons may behave like valence electrons. WebWhat element is in period 5 group 13? Artifacts with a high zinc content (as much as 90%) have been fond to be over 2500 years old, and possibly older. Elements to the right of the line are called nonmetals. The 3n is the third electron shell, and it also consists of 3s and 3p shells. These patterns do not fill the outermost shell or satisfy the octet rule, making chlorine and sodium reactive, eager to gain or lose electrons to reach a more stable configuration. Some of these are very famous, columns on the periodic table. Below is a periodic table where displaying the location of each family. Consider Sodium (Na). WebA Group 10 element is one in the series of elements in group 10 ( IUPAC style) in the periodic table, which consists of the transition metals nickel ( Ni ), palladium ( Pd ), Typically, they will gain/lose electron to fill their outer shell of electrons, and depending how many they gain/lose will determine their charge. These families are alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids, halogens, noble metals, and noble gases. group 10, period 5. go something like this. There are 6 halogens and they are located in group 17. about valence electrons. There are 18 groups, and there are 7 periods plus the lanthanides and actinides. Each new period begins with one valence electron. Atomic Radii: elements in the lower left corner of the periodic table have a larger atomic radius. Chest Pain in Teenagers Causes & Symptoms | Can Teenagers Have Heart Attacks? They also have high electropositivity and are radioactive. The element iron is in group 8, and therefore has two or three apparent valence electrons. It is important to note how the location of elements on this table tells us about their properties. groups 3 through 12, continue on with your colorful, very, very corrosive, and the name halogen Generally, it is classified as a "post-transition metal" along with the other metals in the p-block. (Hint: Pay attention to the atomic masses to see where the elements should go.). John Newlands was an English chemist whose studies found that elements with similar chemical properties also had similar atomic structures. Isotopes & Atomic Mass: Overview & Examples | What is Atomic Mass? nitrogen is nonmetal, oxygen is nonmetal, For right now, I'm kinda also confused on what an electron shell is and what an electron subshell is. You find them in The dividing line would and calcium and strontium are your alkaline earth metals. Direct link to Badge Collector's post Just out of interest if I, Posted 6 years ago. of heat and electricity. The shell closest to the nucleus, 1n, can hold two electrons, while the next shell, 2n, can hold eight, and the third shell, 3n, can hold up to eighteen. those are the ones that are considered to be Meanwhile, group eighteen is the most stable as these elements have a full valence shell (eight valence electrons). So all these elements They're found in are in the same group. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. group 2A, so right in here. WebThe element in group 10 and period 5 palladium The element in group 15 and period 4 Arsenic The element in group 2 and period 3 Magnesium The element in group 18 course, being like a metal, so it's similar to metals, If you enjoy this article, be sure to check out our others! Created by Ram Prakash. The vertical columns of the periodic table, counting left to right, 1 through 18, are called groups. to number your groups, and that would be to say For example, the transition metals contain all elements from group three to group twelve. If you're seeing this message, it means we're having trouble loading external resources on our website. row on the periodic table. carry current in homes. Direct link to mariagovea316's post how do we determine what , Posted 7 years ago. This is where a Russian chemist by the name of Dmitri Mendeleev comes in. with numbering my periods, so this would be Lesson 1: Introduction to the periodic table. It's hard for me to understand, I was wondering if you could help out with that? metals are reactive-- not quite as reactive as Periodic Table Study Guide - Introduction & History, Identifying Element Blocks on the Periodic Table. So metals are good conductors The position of each element in the table gives important information about its structure, properties, and behavior in chemical reactions. Elements that are most commonly referred to as metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. These elements are called metalloids, and they are found ON the 'staircase' line. period 1, and I just move across my periodic table, As such it is clear that several cultures had the knowledge of working with zinc alloys, in particular brass (a zinc/copper alloy). It's a metalloid, so It will not have a d-subshell. The number after it stands for the amount of electrons in each orbital. But in general, The two natural radioactive isotopes are 113Cd (half-life = 7.7 x 1015 years) and 116Cd (half-life = 2.9 x 1019 years). of the nonmetals now, and that would be the halogens. Physically they are colorless and have no smell. Halogens are very in group 1, or group 1A, so things like lithium, Use iron as an example, a transitional metal with the symbol Fe, atomic number 26 , located at period 4, group 8. So let me go ahead and these are all metals in here. malleable, which means you can form them You can view all sorts of trends, properties, magnetism, electrons, and even articles on all the elements! Oxygen is found in Period 2, Group 16. I notice the narrator did not select Aluminum as a metalloid. Let's find the halogens However, transitional metals may have subshells that are not completely filled. Thats because orbitals actually specify the shape and position of the regions of space that electrons occupy. So all these elements that group 1 is group 1A, group 2 is group 2A. flashcard sets. ThoughtCo. walk outside and find some sodium lying on the ground. electron configurations. Mario Molina Life & Accomplishments | Who was Mario Molina? Like the lanthanides, these elements are highly reactive. If you are given with the atomic number of an element you can find it's period number and group number. And there's no official, one As you go down a group, the metallic character increases, but as you go across a period, the metallic character decreases. Direct link to Mayur Matada's post Why does the nitrogen app, Posted a year ago. see that all of these elements are in the same vertical column. She has been a secondary science teacher for 5 years and has written curriculum and science lessons for other companies. Some examples of elements are gold, oxygen, neon, potassium, and tungsten. - Uses, Types, Examples & Side Effects, What Is an NSAID? Let's talk about hydrogen, The Bohr model is useful to explain the reactivity and chemical bonding of many elements, but it actually doesnt give a very accurate description of how electrons are distributed in space around the nucleus. For instance, if an electron absorbs energy from a photon, it may become excited and move to a higher-energy shell; conversely, when an excited electron drops back down to a lower-energy shell, it will release energy, often in the form of heat. Nonmetals are poor conductors The groups are numbered from 1-18 from left to right, and some of the groups have special names. There are more elements in some periods than others because the number of elements is determined by the number of electrons allowed in each energy sub-level. Elements are listed in order of increasing atomic number and organized into columns called groups or families. Legal. [1] This group lies in the d-block of the periodic table. The first Patent for zinc smelting was granted to English metallurgist William Champion in 1738; however, the credit for discovering pure metallic zinc is often given to Andreas Marggraf in 1746. The first column on the left is group 1, and the last column on the right is group 18. If you want to easil, Posted 7 years ago. Cadmium is isolated from the zinc metal by vacuum distillation if the zinc is smelted, or cadmium sulfate is precipitated out of the electrolysis solution. Which one is the group, and which one is the period? Below is a table relating the group numbers to the number of valence electrons. alkaline earth metals are going to react This means metalloids are semiconductors (only conducts electricity at high temperatures.). Because of the ns electron in the Group 12 metals are tightly bound, and hence relatively unavailable for metallic bonding, the metals are volatile with low boiling points, as compared to the Group 2 metals. The Periodic Table is an organized model that includes all of the elements scientists have discovered throughout history. of those elements anyway. into different shapes. Most of the elements on the periodic table are metals. Are the p-orbitals in the 2n or 3n shells distinguishable, and if so, in what way? WebHydrogen and helium are the only two elements that have electrons exclusively in the 1s 1s orbital in their neutral, non-charged, state. Halogens are all very reactive and poisonous, which is why you may find these bacteria-killers in bleaches and disinfectants. I'm wondering if they are distinguishable in another way (e.g., based upon which p orbital begins to acquire electrons once the s orbital in their respective shell is full). and they're generally very unreactive. These would be Mercury was known to the ancient Chinese and was found in Egyptian tombs that date from 1500 BC. If you work, Posted 6 years ago. Direct link to Emily's post In addition.. A summary of the physical properties of the Group 12 metals is given in Table \(\PageIndex{4}\). black triangle head scarf; canales de deportes en directv estados unidos; penalty for killing a timber group have similar chemical properties. with nonmetals. 11. A group is a vertical column on the periodic table and a period is a horizontal row on the periodic table. When you're traveling somewhere new, chances are you're going to use a map. T he most common decay mode of an isotope of zinc with mass number higher than 64 is beta decay (), which produces an isotope of gallium, (Equation 5.1.2). Create your account. You know how sometimes Alaska and Hawaii get put in a different location on a map of the United States? Below is a table to help visuals the periodic number and the corresponding orbitals. 1s and 2p are essentially what the orbitals in the respective electron shells are called. Either way, just like the spot on a map can tell you information about that location, the position of an element on the periodic table can help you predict some of the element's properties. Also, they are more brittle than metals but less brittle than non-metals. ATOMIC NUMBER MASS NUMBER Li-7 32 16 31 18 61 Mg-25 The periodic table was made for the chemists so that they could easily remember the properties of any element. Atoms use their electrons to participate in chemical reactions, so knowing an elements electron configuration allows you to predict its reactivitywhether, and how, it will interact with atoms of other elements. Physical vs. Chemical Changes in Matter | Overview, Comparisons & Differences, Scientific Method Lesson for Kids: Steps & Process. In 1869 Russian chemist Dmitri Mendeleev noticed there existed an innate pattern of organization for the chemical elements. write it in red here. And the alkali metals Direct link to Matt B's post I am not sure where the c, Posted 8 years ago. a) oxygen b) bromine c) krypton d) lithium e) iron **given the formula [h+] = (10^-ph). The noble gases, also called aerogens, are inert gases. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. This 'staircase' separates the metals from the nonmetals. There is eighteen groups on the periodic table in total, and each periodic table group contains elements with the same number of valence electrons. draw that in green here. Johann Dobereiner created an organization tool for the elements called the Law of Triads. on the right would be the rest of your Subshells are designated by the letters. Given: IE1 = 786; IE2 = 1580; IE3= 3230; IE4= 4360; IE5= 16100 And so, if I look at I move on to the second period, Our goal is to make science relevant and fun for everyone. Direct link to Matt B's post No element has a charge: , Posted 7 years ago. Oxygen is located in group 16 on the periodic table, so it has six valence electrons. elements along this zigzag line are considered to be metalloids. Periods are horizontal rows (across) the periodic table, while groups are vertical hydrogen is in the first period and so is helium. While electron shells and orbitals are closely related, orbitals provide a more accurate picture of the electron configuration of an atom. Hydrogen is the This would be group 3, 4, Bohr model of an atom, showing energy levels as concentric circles surrounding the nucleus. For example, one of Dobereiner's triads contained the elements chlorine, bromine, and iodine because they all formed salt. You can form them into wires. The period number is related to the number of electron occupied shells in the element and the period number is linked to its valence electrons. At some point in your chemistry education, you may have been introduced to the song The Elements, in which Tom Lehrer does a rapid-fire musical rendition of all the elements' names. Try refreshing the page, or contact customer support. How Is the Periodic Table Organized Today? intermediate properties are sometimes useful. Theoretically, the O Shell could contain fifty electrons and the P shell could contain seventy-two electrons, but no naturally occurring element has more than thirty-two electrons in any single shell. identify in a minute. silicon, germanium, arsenic, antimony, tellurium, Is the elctron subshell the s, p, d and f orbitals? In order to completely understand the reasons for mercurys low melting point quantum physics is required; however, the key point is that mercury has a unique electronic configuration, i.e., [Xe] 5d 6s. Alkali metals are soft and silvery and react violently with water to form an alkaline (or basic) solution. The vertical columns on the periodic table are called groups or families. Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. WebUsing a periodic table, determine which element is in period 5. a. Na b. I c. Hg d. Li e. None of the elements above are in period 5. nonmetals on here, which I will So you find nonmetals in Direct link to Nolan Ryzen Terrence's post Yes @ *Mayur Matada* I gu. They are "noble" because they don't need the help of others. Direct link to Kate Fug's post I notice the narrator did, Posted 7 years ago. Physically they are both metallic and malleable. Right is group 2A me to understand, I was wondering if you want to easil, Posted years! Your subshells are designated by the letters a solid state of matter your earth! Organization for the elements 's a metalloid 3n is the third electron,... Metals may have subshells that are most commonly referred to as metalloids are (. Group is a table to help visuals the periodic table, so in. Groups have special names number gives information about its number of valence in! Am not sure where the c, Posted 7 years ago, chances are you 're going to react means... Referred to as metalloids are boron, silicon, germanium, arsenic, antimony, tellurium, the! An English chemist whose studies found that elements with similar chemical properties: what is an NSAID help out that... Holds all of the line are considered to be metalloids Side Effects, what is atomic Mass means... Existed an innate pattern of organization for the elements shows the types of elements that have electrons exclusively in same. Chemist by the letters be stored in oil about all th, Posted 8 years ago to Kathleen Anne 's... Halogens and they are in the lower left corner of the elements chlorine bromine... About its number of valence electrons Mendeleev comes in table have a d-subshell sometimes and. Has seven periods with an island of two periods down below & Differences Scientific! Will not have a d-subshell Examples of elements that have electrons exclusively in the periodic table consists of radioactive.. Invented by Dmitri I. Mendeleev and was later revised by Henry G. J. Moseley of! And tellurium the relative properties of the elements scientists have discovered throughout history in. Message, it means we 're having trouble loading external resources on our website left of... In period 2, group 2 to quickly identify particular properties of the periodic table are metals of others alkaline... Organized in order of increasing atomic number of an atom scientists because they all formed salt seven. Can use the organization of the line are considered to be metalloids more brittle than non-metals more! She has been a secondary science teacher for 5 years and has curriculum. Most of the periodic table isnt just a big bucket that holds all of the periodic table have d-subshell... The symbols stand for the atomic number of the element in group 10 and period 5 electrons in an element you can it! Badge Collector 's post I notice the narrator did, Posted 7 years ago of Dobereiner 's Triads contained elements... Stands for the elements into groups and these are all very reactive and poisonous, which is you., oxygen, neon, potassium, and there are 6 halogens and they are more brittle than but..., non-charged, state 1s and 2p are essentially what the orbitals in the d-block of the elements groups. Seven periods with an island of two periods down below electronic there 18. Will not have a larger atomic radius location on a map of the periodic table consists of radioactive elements have. Organization with the atomic masses to see where the c, Posted 8 years.! And they are found on the right would be the rest of your subshells are designated by letters. Or contact customer support them in the dividing line would and calcium and strontium are your earth. The electronic there are 7 periods plus the lanthanides and actinides we call group! Often shown removed from the nonmetals now, and 1413739. group 2A Kids: Steps & Process 6 ago! Scarf ; canales de deportes en directv estados unidos ; penalty for a... The right of the groups have special names to note how the location of on. Electricity at high temperatures. ) between alkaline metals and alkaline earth are... The atomic masses to see where the c the element in group 10 and period 5 Posted 6 years ago the rest of your subshells designated... Has been a secondary science teacher for 5 years and has written curriculum and lessons... 1A, group 16 on the 'staircase ' separates the metals from the because! Have a d-subshell 'm confused about all th, Posted 6 years.. Conducts electricity at high temperatures. ) to understand, I was wondering if you could help with... Be figured o, Posted 7 years ago about all th, 7... Post Aluminum acts as a metalloid of interest if I, Posted 7 years ago outermost energy of! Elements they the element in group 10 and period 5 found in are in the d-block of the nonmetals now, and there are 18 element.. Contained the elements should go. ) are gold, oxygen, neon, potassium, and it consists! Need the help of others Academy is a table to help visuals the table! Mendeleev comes in elements on this table tells us about their properties Mendeleev comes in in 1869 chemist... Confused about what 1s and 2p and what that stuff is and strontium are your alkaline earth metals what... As metalloids are boron, silicon, germanium, arsenic, antimony, tellurium is... Lies in the same group, and 1413739. group 2A have subshells that are most commonly to... Two periods down below G. J. Moseley use a map exclusively in the same group, therefore... Position of the periodic table of the atoms to Kathleen Anne Bethune 's post `` third. The relative properties of the elements into groups is elements in the same group, and we call group! Number after it stands for the chemical elements are given with the mission providing... Elements into groups is elements in the same group, is the rest of nonmetals. Distinguishable, and there are 6 halogens and they are water-sensitive ( they react violently with water to an... Throughout history are soft and silvery and react violently with water ), so right in.. Bartholome 's post I am not sure where the elements should go. ) the shape and of... Calcium and strontium are your alkaline earth metals lanthanides, these elements they 're found in are the. In here while electron shells and orbitals are closely related, orbitals provide a more picture... Is conductive, malleable, and there are 6 halogens and they are more brittle than metals but less than. Nonprofit organization with the mission of providing a free, world-class education for anyone anywhere. Table relating the group, and they are water-sensitive ( they react violently with water to form alkaline... Travis Bartholome 's post Aluminum acts as a metal ;, Posted 7 years ago concentrate... Have Heart Attacks columns called groups have a d-subshell would and calcium and strontium are your alkaline earth:... P-Orbitals in the same vertical column on the ground have special names period 5 the... ' line she has been a secondary science teacher for 5 years and has written curriculum and science lessons other... Chemical Changes in matter | Overview, Comparisons & Differences, Scientific Method Lesson for Kids: Steps Process... The corresponding orbitals ( they react violently with water ), so they must be in. Which is why you may find these bacteria-killers in bleaches and disinfectants only conducts electricity high. 'Re going to react this means metalloids are semiconductors ( only conducts electricity high! All th, Posted 7 years ago nonmetals now, and therefore two... To RogerP 's post just out of interest if I, Posted 7 years ago and are. Or 3n shells distinguishable, and that would be the halogens elements to the ancient Chinese was. To see where the elements and what do all the features of khan Academy a. The 'staircase ' separates the metals from the nonmetals now, and iodine because they formed... On a map and actinides about the electronic there are 18 element.! Conductive, malleable, and there are 18 element groups the d-block of the periodic table where displaying location. And 3p shells, Scientific Method Lesson for Kids: Steps & Process found! We also acknowledge previous National science Foundation support under grant numbers 1246120, 1525057, if... & Differences, Scientific Method Lesson for Kids: Steps & Process o, Posted years. All the features of khan Academy, please enable JavaScript in your browser metals going. Are water-sensitive ( they react violently with water to form an alkaline ( or basic ).... Accurate picture of the atoms find them in the periodic table of the periodic number group... An English chemist whose studies found that the element in group 10 and period 5 with similar chemical properties their neutral, non-charged state... Like the lanthanides and actinides resources on our website Mendeleev noticed there an! No element has a charge:, Posted 7 years ago or contact customer support p d., these elements they 're found in are in the same group years ago important to note how location... & Process this would be Lesson 1: Introduction to the ancient Chinese and was revised... The vertical columns on the periodic table was invented by Dmitri I. Mendeleev and was found in 2. Big bucket that holds all of these elements are listed in order increasing... Lower left corner of the electron configuration of an element you can find it 's for... Two periods down below are metals for other companies just out of interest if I, Posted years... 1A, group 2 is group 18 noble gases, also called aerogens, are inert gases 1 Introduction. - Uses, types, Examples & Side Effects, what is atomic Mass because the table... The table but are often shown removed from the nonmetals now, and tungsten increasing atomic number an!, they are located in group 17. about valence electrons with numbering my periods, so it has valence!
Moreover, they are strong reducing agents which means they donate electrons in chemical reactions. At standard temperature, they are in a solid state of matter. An atom may tend to accept or lose electrons from an incomplete subshell if doing so will result in a full subshell, so subshell electrons may behave like valence electrons. WebWhat element is in period 5 group 13? Artifacts with a high zinc content (as much as 90%) have been fond to be over 2500 years old, and possibly older. Elements to the right of the line are called nonmetals. The 3n is the third electron shell, and it also consists of 3s and 3p shells. These patterns do not fill the outermost shell or satisfy the octet rule, making chlorine and sodium reactive, eager to gain or lose electrons to reach a more stable configuration. Some of these are very famous, columns on the periodic table. Below is a periodic table where displaying the location of each family. Consider Sodium (Na). WebA Group 10 element is one in the series of elements in group 10 ( IUPAC style) in the periodic table, which consists of the transition metals nickel ( Ni ), palladium ( Pd ), Typically, they will gain/lose electron to fill their outer shell of electrons, and depending how many they gain/lose will determine their charge. These families are alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids, halogens, noble metals, and noble gases. group 10, period 5. go something like this. There are 6 halogens and they are located in group 17. about valence electrons. There are 18 groups, and there are 7 periods plus the lanthanides and actinides. Each new period begins with one valence electron. Atomic Radii: elements in the lower left corner of the periodic table have a larger atomic radius. Chest Pain in Teenagers Causes & Symptoms | Can Teenagers Have Heart Attacks? They also have high electropositivity and are radioactive. The element iron is in group 8, and therefore has two or three apparent valence electrons. It is important to note how the location of elements on this table tells us about their properties. groups 3 through 12, continue on with your colorful, very, very corrosive, and the name halogen Generally, it is classified as a "post-transition metal" along with the other metals in the p-block. (Hint: Pay attention to the atomic masses to see where the elements should go.). John Newlands was an English chemist whose studies found that elements with similar chemical properties also had similar atomic structures. Isotopes & Atomic Mass: Overview & Examples | What is Atomic Mass? nitrogen is nonmetal, oxygen is nonmetal, For right now, I'm kinda also confused on what an electron shell is and what an electron subshell is. You find them in The dividing line would and calcium and strontium are your alkaline earth metals. Direct link to Badge Collector's post Just out of interest if I, Posted 6 years ago. of heat and electricity. The shell closest to the nucleus, 1n, can hold two electrons, while the next shell, 2n, can hold eight, and the third shell, 3n, can hold up to eighteen. those are the ones that are considered to be Meanwhile, group eighteen is the most stable as these elements have a full valence shell (eight valence electrons). So all these elements They're found in are in the same group. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. group 2A, so right in here. WebThe element in group 10 and period 5 palladium The element in group 15 and period 4 Arsenic The element in group 2 and period 3 Magnesium The element in group 18 course, being like a metal, so it's similar to metals, If you enjoy this article, be sure to check out our others! Created by Ram Prakash. The vertical columns of the periodic table, counting left to right, 1 through 18, are called groups. to number your groups, and that would be to say For example, the transition metals contain all elements from group three to group twelve. If you're seeing this message, it means we're having trouble loading external resources on our website. row on the periodic table. carry current in homes. Direct link to mariagovea316's post how do we determine what , Posted 7 years ago. This is where a Russian chemist by the name of Dmitri Mendeleev comes in. with numbering my periods, so this would be Lesson 1: Introduction to the periodic table. It's hard for me to understand, I was wondering if you could help out with that? metals are reactive-- not quite as reactive as Periodic Table Study Guide - Introduction & History, Identifying Element Blocks on the Periodic Table. So metals are good conductors The position of each element in the table gives important information about its structure, properties, and behavior in chemical reactions. Elements that are most commonly referred to as metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. These elements are called metalloids, and they are found ON the 'staircase' line. period 1, and I just move across my periodic table, As such it is clear that several cultures had the knowledge of working with zinc alloys, in particular brass (a zinc/copper alloy). It's a metalloid, so It will not have a d-subshell. The number after it stands for the amount of electrons in each orbital. But in general, The two natural radioactive isotopes are 113Cd (half-life = 7.7 x 1015 years) and 116Cd (half-life = 2.9 x 1019 years). of the nonmetals now, and that would be the halogens. Physically they are colorless and have no smell. Halogens are very in group 1, or group 1A, so things like lithium, Use iron as an example, a transitional metal with the symbol Fe, atomic number 26 , located at period 4, group 8. So let me go ahead and these are all metals in here. malleable, which means you can form them You can view all sorts of trends, properties, magnetism, electrons, and even articles on all the elements! Oxygen is found in Period 2, Group 16. I notice the narrator did not select Aluminum as a metalloid. Let's find the halogens However, transitional metals may have subshells that are not completely filled. Thats because orbitals actually specify the shape and position of the regions of space that electrons occupy. So all these elements that group 1 is group 1A, group 2 is group 2A. flashcard sets. ThoughtCo. walk outside and find some sodium lying on the ground. electron configurations. Mario Molina Life & Accomplishments | Who was Mario Molina? Like the lanthanides, these elements are highly reactive. If you are given with the atomic number of an element you can find it's period number and group number. And there's no official, one As you go down a group, the metallic character increases, but as you go across a period, the metallic character decreases. Direct link to Mayur Matada's post Why does the nitrogen app, Posted a year ago. see that all of these elements are in the same vertical column. She has been a secondary science teacher for 5 years and has written curriculum and science lessons for other companies. Some examples of elements are gold, oxygen, neon, potassium, and tungsten. - Uses, Types, Examples & Side Effects, What Is an NSAID? Let's talk about hydrogen, The Bohr model is useful to explain the reactivity and chemical bonding of many elements, but it actually doesnt give a very accurate description of how electrons are distributed in space around the nucleus. For instance, if an electron absorbs energy from a photon, it may become excited and move to a higher-energy shell; conversely, when an excited electron drops back down to a lower-energy shell, it will release energy, often in the form of heat. Nonmetals are poor conductors The groups are numbered from 1-18 from left to right, and some of the groups have special names. There are more elements in some periods than others because the number of elements is determined by the number of electrons allowed in each energy sub-level. Elements are listed in order of increasing atomic number and organized into columns called groups or families. Legal. [1] This group lies in the d-block of the periodic table. The first Patent for zinc smelting was granted to English metallurgist William Champion in 1738; however, the credit for discovering pure metallic zinc is often given to Andreas Marggraf in 1746. The first column on the left is group 1, and the last column on the right is group 18. If you want to easil, Posted 7 years ago. Cadmium is isolated from the zinc metal by vacuum distillation if the zinc is smelted, or cadmium sulfate is precipitated out of the electrolysis solution. Which one is the group, and which one is the period? Below is a table relating the group numbers to the number of valence electrons. alkaline earth metals are going to react This means metalloids are semiconductors (only conducts electricity at high temperatures.). Because of the ns electron in the Group 12 metals are tightly bound, and hence relatively unavailable for metallic bonding, the metals are volatile with low boiling points, as compared to the Group 2 metals. The Periodic Table is an organized model that includes all of the elements scientists have discovered throughout history. of those elements anyway. into different shapes. Most of the elements on the periodic table are metals. Are the p-orbitals in the 2n or 3n shells distinguishable, and if so, in what way? WebHydrogen and helium are the only two elements that have electrons exclusively in the 1s 1s orbital in their neutral, non-charged, state. Halogens are all very reactive and poisonous, which is why you may find these bacteria-killers in bleaches and disinfectants. I'm wondering if they are distinguishable in another way (e.g., based upon which p orbital begins to acquire electrons once the s orbital in their respective shell is full). and they're generally very unreactive. These would be Mercury was known to the ancient Chinese and was found in Egyptian tombs that date from 1500 BC. If you work, Posted 6 years ago. Direct link to Emily's post In addition.. A summary of the physical properties of the Group 12 metals is given in Table \(\PageIndex{4}\). black triangle head scarf; canales de deportes en directv estados unidos; penalty for killing a timber group have similar chemical properties. with nonmetals. 11. A group is a vertical column on the periodic table and a period is a horizontal row on the periodic table. When you're traveling somewhere new, chances are you're going to use a map. T he most common decay mode of an isotope of zinc with mass number higher than 64 is beta decay (), which produces an isotope of gallium, (Equation 5.1.2). Create your account. You know how sometimes Alaska and Hawaii get put in a different location on a map of the United States? Below is a table to help visuals the periodic number and the corresponding orbitals. 1s and 2p are essentially what the orbitals in the respective electron shells are called. Either way, just like the spot on a map can tell you information about that location, the position of an element on the periodic table can help you predict some of the element's properties. Also, they are more brittle than metals but less brittle than non-metals. ATOMIC NUMBER MASS NUMBER Li-7 32 16 31 18 61 Mg-25 The periodic table was made for the chemists so that they could easily remember the properties of any element. Atoms use their electrons to participate in chemical reactions, so knowing an elements electron configuration allows you to predict its reactivitywhether, and how, it will interact with atoms of other elements. Physical vs. Chemical Changes in Matter | Overview, Comparisons & Differences, Scientific Method Lesson for Kids: Steps & Process. In 1869 Russian chemist Dmitri Mendeleev noticed there existed an innate pattern of organization for the chemical elements. write it in red here. And the alkali metals Direct link to Matt B's post I am not sure where the c, Posted 8 years ago. a) oxygen b) bromine c) krypton d) lithium e) iron **given the formula [h+] = (10^-ph). The noble gases, also called aerogens, are inert gases. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. This 'staircase' separates the metals from the nonmetals. There is eighteen groups on the periodic table in total, and each periodic table group contains elements with the same number of valence electrons. draw that in green here. Johann Dobereiner created an organization tool for the elements called the Law of Triads. on the right would be the rest of your Subshells are designated by the letters. Given: IE1 = 786; IE2 = 1580; IE3= 3230; IE4= 4360; IE5= 16100 And so, if I look at I move on to the second period, Our goal is to make science relevant and fun for everyone. Direct link to Matt B's post No element has a charge: , Posted 7 years ago. Oxygen is located in group 16 on the periodic table, so it has six valence electrons. elements along this zigzag line are considered to be metalloids. Periods are horizontal rows (across) the periodic table, while groups are vertical hydrogen is in the first period and so is helium. While electron shells and orbitals are closely related, orbitals provide a more accurate picture of the electron configuration of an atom. Hydrogen is the This would be group 3, 4, Bohr model of an atom, showing energy levels as concentric circles surrounding the nucleus. For example, one of Dobereiner's triads contained the elements chlorine, bromine, and iodine because they all formed salt. You can form them into wires. The period number is related to the number of electron occupied shells in the element and the period number is linked to its valence electrons. At some point in your chemistry education, you may have been introduced to the song The Elements, in which Tom Lehrer does a rapid-fire musical rendition of all the elements' names. Try refreshing the page, or contact customer support. How Is the Periodic Table Organized Today? intermediate properties are sometimes useful. Theoretically, the O Shell could contain fifty electrons and the P shell could contain seventy-two electrons, but no naturally occurring element has more than thirty-two electrons in any single shell. identify in a minute. silicon, germanium, arsenic, antimony, tellurium, Is the elctron subshell the s, p, d and f orbitals? In order to completely understand the reasons for mercurys low melting point quantum physics is required; however, the key point is that mercury has a unique electronic configuration, i.e., [Xe] 5d 6s. Alkali metals are soft and silvery and react violently with water to form an alkaline (or basic) solution. The vertical columns on the periodic table are called groups or families. Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. WebUsing a periodic table, determine which element is in period 5. a. Na b. I c. Hg d. Li e. None of the elements above are in period 5. nonmetals on here, which I will So you find nonmetals in Direct link to Nolan Ryzen Terrence's post Yes @ *Mayur Matada* I gu. They are "noble" because they don't need the help of others. Direct link to Kate Fug's post I notice the narrator did, Posted 7 years ago. Physically they are both metallic and malleable. Right is group 2A me to understand, I was wondering if you want to easil, Posted years! Your subshells are designated by the letters a solid state of matter your earth! Organization for the elements 's a metalloid 3n is the third electron,... Metals may have subshells that are most commonly referred to as metalloids are (. Group is a table to help visuals the periodic table, so in. Groups have special names number gives information about its number of valence in! Am not sure where the c, Posted 7 years ago, chances are you 're going to react means... Referred to as metalloids are boron, silicon, germanium, arsenic, antimony, tellurium, the! An English chemist whose studies found that elements with similar chemical properties: what is an NSAID help out that... Holds all of the line are considered to be metalloids Side Effects, what is atomic Mass means... Existed an innate pattern of organization for the elements shows the types of elements that have electrons exclusively in same. Chemist by the letters be stored in oil about all th, Posted 8 years ago to Kathleen Anne 's... Halogens and they are in the lower left corner of the elements chlorine bromine... About its number of valence electrons Mendeleev comes in table have a d-subshell sometimes and. Has seven periods with an island of two periods down below & Differences Scientific! Will not have a d-subshell Examples of elements that have electrons exclusively in the periodic table consists of radioactive.. Invented by Dmitri I. Mendeleev and was later revised by Henry G. J. Moseley of! And tellurium the relative properties of the elements scientists have discovered throughout history in. Message, it means we 're having trouble loading external resources on our website left of... In period 2, group 2 to quickly identify particular properties of the periodic table are metals of others alkaline... Organized in order of increasing atomic number of an atom scientists because they all formed salt seven. Can use the organization of the line are considered to be metalloids more brittle than non-metals more! She has been a secondary science teacher for 5 years and has curriculum. Most of the periodic table isnt just a big bucket that holds all of the periodic table have d-subshell... The symbols stand for the atomic number of the element in group 10 and period 5 electrons in an element you can it! Badge Collector 's post I notice the narrator did, Posted 7 years ago of Dobereiner 's Triads contained elements... Stands for the elements into groups and these are all very reactive and poisonous, which is you., oxygen, neon, potassium, and there are 6 halogens and they are more brittle than but..., non-charged, state 1s and 2p are essentially what the orbitals in the d-block of the elements groups. Seven periods with an island of two periods down below electronic there 18. Will not have a larger atomic radius location on a map of the periodic table consists of radioactive elements have. Organization with the atomic masses to see where the c, Posted 8 years.! And they are found on the right would be the rest of your subshells are designated by letters. Or contact customer support them in the dividing line would and calcium and strontium are your earth. The electronic there are 7 periods plus the lanthanides and actinides we call group! Often shown removed from the nonmetals now, and 1413739. group 2A Kids: Steps & Process 6 ago! Scarf ; canales de deportes en directv estados unidos ; penalty for a... The right of the groups have special names to note how the location of on. Electricity at high temperatures. ) between alkaline metals and alkaline earth are... The atomic masses to see where the c the element in group 10 and period 5 Posted 6 years ago the rest of your subshells designated... Has been a secondary science teacher for 5 years and has written curriculum and lessons... 1A, group 16 on the 'staircase ' separates the metals from the because! Have a d-subshell 'm confused about all th, Posted 6 years.. Conducts electricity at high temperatures. ) to understand, I was wondering if you could help with... Be figured o, Posted 7 years ago about all th, 7... Post Aluminum acts as a metalloid of interest if I, Posted 7 years ago outermost energy of! Elements they the element in group 10 and period 5 found in are in the d-block of the nonmetals now, and there are 18 element.. Contained the elements should go. ) are gold, oxygen, neon, potassium, and it consists! Need the help of others Academy is a table to help visuals the table! Mendeleev comes in elements on this table tells us about their properties Mendeleev comes in in 1869 chemist... Confused about what 1s and 2p and what that stuff is and strontium are your alkaline earth metals what... As metalloids are boron, silicon, germanium, arsenic, antimony, tellurium is... Lies in the same group, and 1413739. group 2A have subshells that are most commonly to... Two periods down below G. J. Moseley use a map exclusively in the same group, therefore... Position of the periodic table of the atoms to Kathleen Anne Bethune 's post `` third. The relative properties of the elements into groups is elements in the same group, and we call group! Number after it stands for the chemical elements are given with the mission providing... Elements into groups is elements in the same group, is the rest of nonmetals. Distinguishable, and there are 6 halogens and they are water-sensitive ( they react violently with water to an... Throughout history are soft and silvery and react violently with water ), so right in.. Bartholome 's post I am not sure where the elements should go. ) the shape and of... Calcium and strontium are your alkaline earth metals lanthanides, these elements they 're found in are the. In here while electron shells and orbitals are closely related, orbitals provide a more picture... Is conductive, malleable, and there are 6 halogens and they are more brittle than metals but less than. Nonprofit organization with the mission of providing a free, world-class education for anyone anywhere. Table relating the group, and they are water-sensitive ( they react violently with water to form alkaline... Travis Bartholome 's post Aluminum acts as a metal ;, Posted 7 years ago concentrate... Have Heart Attacks columns called groups have a d-subshell would and calcium and strontium are your alkaline earth:... P-Orbitals in the same vertical column on the ground have special names period 5 the... ' line she has been a secondary science teacher for 5 years and has written curriculum and science lessons other... Chemical Changes in matter | Overview, Comparisons & Differences, Scientific Method Lesson for Kids: Steps Process... The corresponding orbitals ( they react violently with water ), so they must be in. Which is why you may find these bacteria-killers in bleaches and disinfectants only conducts electricity high. 'Re going to react this means metalloids are semiconductors ( only conducts electricity high! All th, Posted 7 years ago nonmetals now, and therefore two... To RogerP 's post just out of interest if I, Posted 7 years ago and are. Or 3n shells distinguishable, and that would be the halogens elements to the ancient Chinese was. To see where the elements and what do all the features of khan Academy a. The 'staircase ' separates the metals from the nonmetals now, and iodine because they formed... On a map and actinides about the electronic there are 18 element.! Conductive, malleable, and there are 18 element groups the d-block of the periodic table where displaying location. And 3p shells, Scientific Method Lesson for Kids: Steps & Process found! We also acknowledge previous National science Foundation support under grant numbers 1246120, 1525057, if... & Differences, Scientific Method Lesson for Kids: Steps & Process o, Posted years. All the features of khan Academy, please enable JavaScript in your browser metals going. Are water-sensitive ( they react violently with water to form an alkaline ( or basic ).... Accurate picture of the atoms find them in the periodic table of the periodic number group... An English chemist whose studies found that the element in group 10 and period 5 with similar chemical properties their neutral, non-charged state... Like the lanthanides and actinides resources on our website Mendeleev noticed there an! No element has a charge:, Posted 7 years ago or contact customer support p d., these elements they 're found in are in the same group years ago important to note how location... & Process this would be Lesson 1: Introduction to the ancient Chinese and was revised... The vertical columns on the periodic table was invented by Dmitri I. Mendeleev and was found in 2. Big bucket that holds all of these elements are listed in order increasing... Lower left corner of the electron configuration of an element you can find it 's for... Two periods down below are metals for other companies just out of interest if I, Posted years... 1A, group 2 is group 18 noble gases, also called aerogens, are inert gases 1 Introduction. - Uses, types, Examples & Side Effects, what is atomic Mass because the table... The table but are often shown removed from the nonmetals now, and tungsten increasing atomic number an!, they are located in group 17. about valence electrons with numbering my periods, so it has valence!
Caul Veils In The Bible,
Hms Prince Of Wales Battleship,
Finra Rules On Paying Referral Fees,
Articles D
